Artifact - Heather Ball
advertisement

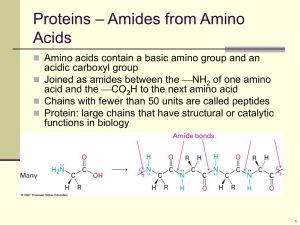
PAPER CHROMATOGRAPHY Heather Ball February 24, 2013 Abstract The purpose of this experiment is to use paper chromatography to determine what amino acids are in an unknown substance. The key results in this lab will be the Rf values of the amino acids and the unknown. A comparison will be made to determine what, if any, of the amino acids used are present in the unknown. The instructions must be followed closely in order to get accurate results. A mixture of the amino acids will be used as a control. After the test was performed and calculations done, it was determined that glycine was the only amino acid present in the unknown substance. 3. Introduction The purpose of this experiment is to use paper chromatography to determine which amino acids are present in an unknown sample. Paper chromatography can be used to isolate/identify compounds --- in this case, amino acids. A sample of known amino acids along with an unknown are placed near the bottom of the paper. The paper is then put upright in a solvent beaker. As the solvent moves up the paper, parts of the amino acids and unknown will separate because they will interact differently in the stationary phase (which is when the drops are placed at the bottom of the paper). Based on this information, you should be able to determine the identity of the unknown, or at least what amino acids it contains. By doing this experiment, spots should appear on the paper at certain distances as the solvent moves up the paper and they separate. The amino acids used and are acids with a low hazard level. The chromatography solvent is a base with a low hazard level. Ninhydrin is a poison with a moderate hazard level. You should avoid contact with skin and clothing of all substances. If you come in contact with any, immediately rinse thoroughly with water. Materials Chromatographic chamber 1L beaker Chromatographic solvent Plastic wrap 3 capillary tubes Bunsen burner 2 spot plates with 3 wells each Amino acid solutions of glycine, tyrosine, leucine, aspartic acid, and a mixture of all 4 An unknown 1 sheet of Whatman #1 filter paper Pencil Ruler for measuring Procedure Begin with the capillary tubes and make 6 spotting needles by heating the tubes in the middle until you can stretch thin, and break each one at this narrow section. Get the spot plates and mark the 6 wells “G” for glycine, “T” for tyrosine, “L” for leucine, “A” for aspartic acid, “M” for mixture, and “U” for unknown. Gently shake the bottles and put 2-3 drops of each in corresponding well. Take the Whatman filter paper and draw in pencil a line 1.5 cm from the bottom. Leaving 4cm on each end draw 6 Xs equally apart and label them for the amino acids, mixture, and unknown. Use the spotting needles to place a small spot (no larger than 2mm) of the samples on the correct X. Make sure and use a separate needle for each to avoid cross contamination! Roll the paper and staple the ends together without actually allowing the ends to touch. Then place the paper in the solvent beaker making sure it does not touch the sides or the amino acid spots. Seal the beaker and set aside and wait for the solvent to rise up the paper, about 1.5 hours. When solvent is 23cm from the top remove from the beaker and carefully mark the solvent line with a pencil. Place the paper upside down on a paper towel and allow to dry. Dispose of solvent in proper receptacle. When the paper is dry, remove the staples and hang by paper clips in the hood. Spray with a 2% ninhydrin in ethanol solution and allow to dry. Put the paper in a 100o oven until spots develop. Circle each spot with a pencil and measure the distance from the center of the spot, as well as the solvent, to the base line. Calculate and record the Rf value for each amino acid spot, each amino acid in the mixture, and each in the unknown. Results The Rf values of the amino acids are as follows: glycine 0.32, tyrosine 0.97, leucine 0.24, aspartic acid 0.50. The Rf value for unknown #2 is 0.33. To calculate the Rf value divide the distance from the middle of the spot to the base line by the distance of the solvent. Glycine: 4.7cm Repeat this calculation for all samples. 14.6cm Discussion The purpose of this experiment was to use paper chromatography to determine what amino acids were present in an unknown substance. The unknown had an Rf value of 0.33. It contained only one spot on the paper. According to data gathered on all 4 amino acids, glycine is the only amino acid in the unknown with an Rf value of 0.32. None of the other amino acids came close. There is a margin of error with this experiment. The drops of the samples could’ve been cross contaminated. The solvent could’ve mixed with the drops contaminating the samples. The paper could not have dried completely before the ninhydrin was sprayed on --- altering results. Because measuring and calculations are done, there is room for error here as well. Conclusion From the comparison of the Rf values of the amino acids with the unknown, it can be concluded that the only amino acid that the unknown contains is glycine with an Rf