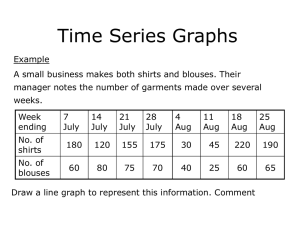
DOCX format - 372 KB - Office of the Gene Technology Regulator
advertisement

Risk Assessment and Risk Management Plan for DIR 130 Limited and controlled release of wheat genetically modified for improved grain quality Applicant: Murdoch University March 2015 PAGE INTENTIONALLY LEFT BLANK DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Summary of the Risk Assessment and Risk Management Plan for Licence Application DIR 130 Decision The Gene Technology Regulator (the Regulator) has decided to issue a licence application for the intentional release of a genetically modified organism (GMO) into the environment. A Risk Assessment and Risk Management Plan (RARMP) for this application was prepared by the Regulator in accordance with requirements of the Gene Technology Act 2000 (the Act) and corresponding state and territory legislation, and finalised following consultation with a wide range of experts, agencies and authorities, and the public. The RARMP concludes that this field trial poses negligible risks to human health and safety and the environment and that any risks posed by the dealings can be managed by imposing conditions on the release. The application Application number DIR 130 Applicant: Murdoch University Project Title: Limited and controlled release of wheat genetically modified for improved grain quality1 Parent organism: Introduced genes and modified traits: Bread wheat (Triticum aestivum L.) Dx5 and Dy10 genes from wheat (improved grain quality) bar gene from Streptomyces hygroscopicus (selectable marker – herbicide tolerance) Proposed release dates: May 2015 – December 2017 Proposed location: One site in the local government area of Katanning, Western Australia 600 square metres each year Proposed release size: Primary purpose To assess whether the introduction and expression of the genes will increase the strength of dough Risk assessment The risk assessment concludes that there are negligible risks to the health and safety of people, or the environment, from the proposed release. No additional controls are required to manage these neglible risks beyond those proposed in the application. The risk assessment process considers how the genetic modification and activities conducted with the GMOs might lead to harm to people or the environment. Risks are characterised in relation to both the seriousness and likelihood of harm, taking into account information in the application (including proposed limits and controls), relevant previous approvals and current scientific/technical knowledge, and advice received from a wide range of experts, agencies and authorities consulted on the RARMP. Both the short and long term potential harms are considered. The title of the project as supplied by the applicant is ‘Field trial of glutenin transgenic wheat for grain quality improvement’. 1 Summary I DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Credible pathways to potential harm that were considered included: unintended exposure to the GM plant material; increased spread and persistence of the GM wheat relative to unmodified plants; and transfer of the introduced genetic material to non GM wheat, or other sexually compatible plants. Potential harms associated with these pathways included toxicity to people and other animals, allergic reactions in people and environmental harms associated with weediness. The principal reasons for the conclusion of negligible risks are that the introduced genes are either identical, or similar, to those already existing in the environment (some of these genes are present in unmodified wheat); and the proposed limits and controls effectively contain the GMOs and their genetic material and minimise exposure. Risk management plan The risk management plan describes measures to protect the health and safety of people and to protect the environment by controlling or mitigating risk. The risk management plan is given effect through licence conditions. As the level of risk is considered negligible, specific risk treatment is not required. However, as this is a limited and controlled release, the licence includes limits on the size, location and duration of the release, as well as controls including containment provisions at the trial site; prohibiting the use of GM plant materials in human food or animal feed; destroying GM plant materials not required for further studies; transporting GM plant materials in accordance with the Regulator’s guidelines; and conducting post-harvest monitoring at the trial site to ensure all GMOs are destroyed. Summary II DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Table of Contents SUMMARY OF THE RISK ASSESSMENT AND RISK MANAGEMENT PLAN ................................................... I Decision .......................................................................................................................................................................... I The application................................................................................................................................................................ I Risk assessment .............................................................................................................................................................. I Risk management plan .................................................................................................................................................. II TABLE OF CONTENTS ...............................................................................................................................................III ABBREVIATIONS ......................................................................................................................................................... IV CHAPTER 1 RISK ASSESSMENT CONTEXT.................................................................................................... 1 Section 1 Section 2 Section 3 3.1 3.2 5.4 Section 6 6.1 6.2 6.3 6.4 Section 7 7.1 7.2 Background .......................................................................................................................................... 1 Regulatory framework ......................................................................................................................... 1 The proposed dealings ......................................................................................................................... 2 The proposed limits of the dealings (size, location, duration and people) ........................................... 2 The proposed controls to restrict the spread and persistence of the GMOs and their genetic material in the environment ............................................................................................................................... 3 The parent organism ............................................................................................................................ 4 The GMOs, nature and effect of the genetic modification ................................................................... 4 Introduction to the GMOs .................................................................................................................... 4 The introduced genes, encoded proteins and their associated effects .................................................. 5 Toxicity/allergenicity or other adverse effects upon health associated with the introduced genes, their encoded proteins and associated products ................................................................................... 6 Characterisation of the GMOs ............................................................................................................. 7 The receiving environment .................................................................................................................. 8 Relevant abiotic factors ....................................................................................................................... 8 Relevant agricultural practices ............................................................................................................. 8 Presence of related plants in the receiving environment ...................................................................... 8 Presence of similar genes and encoded proteins in the environment ................................................... 8 Relevant Australian and international approvals ................................................................................. 9 Australian approvals ............................................................................................................................ 9 International approvals of GM wheat .................................................................................................. 9 CHAPTER 2 RISK ASSESSMENT ...................................................................................................................... 10 Section 1 Section 2 2.1 2.2 2.3 2.4 Section 3 Section 4 Introduction ....................................................................................................................................... 10 Risk Identification ............................................................................................................................. 11 Risk source ........................................................................................................................................ 11 Causal pathway .................................................................................................................................. 12 Potential harm .................................................................................................................................... 13 Postulated risk scenarios .................................................................................................................... 13 Uncertainty ........................................................................................................................................ 26 Risk evaluation .................................................................................................................................. 27 CHAPTER 3 RISK MANAGEMENT .................................................................................................................. 29 Section 1 Section 2 Section 3 3.1 3.2 Section 4 Section 5 Background ........................................................................................................................................ 29 Risk treatment measures for identified risks ...................................................................................... 29 General risk management .................................................................................................................. 29 Licence conditions to limit and control the release ............................................................................ 29 Other risk management considerations .............................................................................................. 32 Issues to be addressed for future releases .......................................................................................... 34 Conclusions of the RARMP .............................................................................................................. 34 REFERENCES ........................................................................................................................................................... 35 APPENDIX A SUMMARY OF SUBMISSIONS FROM PRESCRIBED EXPERTS, AGENCIES AND AUTHORITIES ............................................................................................................................... 44 APPENDIX B SUMMARY OF SUBMISSIONS FROM THE PUBLIC ............................................................. 45 Section 4 Section 5 5.1 5.2 5.3 Table of Contents III DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Abbreviations APVMA CCI Australian Pesticides and Veterinary Medicines Authority Confidential Commercial Information as declared under section 185 of the Gene Technology Act 2000 DAFWA DIR FSANZ GM GMO ha HMW-GS HMW-GSs LGA LMW-GS LMW-GSs m NGNE NLRD OGTR PC2 ppm RARMP Regulations Regulator TGA the Act Department of Agriculture and Food, Western Australia Dealings involving Intentional Release Food Standards Australia New Zealand Genetically modified Genetically modified organism Hectare High molecular weight glutenin subunit High molecular weight glutenin subunits Local government area Low molecular weight glutenin subunit Low molecular weight glutenin subunits Metres New Genes for New Environments Notifiable low risk dealings Office of the Gene Technology Regulator Physical Containment level 2 Parts per million Risk Assessment and Risk Management Plan Gene Technology Regulations 2001 Gene Technology Regulator Therapeutic Goods Administration The Gene Technology Act 2000 Abbreviations IV DIR 130 – Risk Assessment and Risk Management Plan Chapter 1 Office of the Gene Technology Regulator Risk assessment context Section 1 Background An application has been made under the Gene Technology Act 2000 (the Act) for Dealings involving the Intentional Release (DIR) of genetically modified organisms (GMOs) into the Australian environment. The Act in conjunction with the Gene Technology Regulations 2001 (the Regulations), an inter-governmental agreement and corresponding legislation that is being enacted in each State and Territory, comprise Australia’s national regulatory system for gene technology. Its objective is to protect the health and safety of people, and to protect the environment, by identifying risks posed by or as a result of gene technology, and by managing those risks through regulating certain dealings with genetically modified organisms (GMOs). This chapter describes the parameters within which potential risks to the health and safety of people or the environment posed by the proposed release are assessed. The risk assessment context is established within the regulatory framework and considers application-specific parameters (Figure 1). RISK ASSESSMENT CONTEXT LEGISLATIVE REQUIREMENTS (including Gene Technology Act and Regulations) RISK ANALYSIS FRAMEWORK OGTR OPERATIONAL POLICIES AND GUIDELINES PROPOSED DEALINGS Proposed activities involving the GMO Proposed limits of the release Proposed control measures GMO Introduced genes (genotype) Novel traits (phenotype) PREVIOUS RELEASES PARENT ORGANISM Origin and taxonomy Cultivation and use Biological characterisation Ecology RECEIVING ENVIRONMENT Environmental conditions Agronomic practices Presence of related species Presence of similar genes Figure 1 Summary of parameters used to establish the risk assessment context Section 2 Regulatory framework Sections 50, 50A and 51 of the Act outline the matters that the Gene Technology Regulator (the Regulator) must take into account, and who must be consulted with, in preparing the Risk Assessment and Risk Management Plans (RARMPs) that inform the decisions on licence applications. In addition, the Regulations outline further matters the Regulator must consider when preparing a RARMP. In accordance with section 50A of the Gene Technology Act 2000 (the Act), this application is considered to be a limited and controlled release application, as its principal purpose is to enable the applicant to conduct experiments and the applicant has proposed limits on the size, location and duration of the release, as well as controls to restrict the spread and persistence of the GMOs and their genetic material in the environment. Therefore, the Gene Technology Regulator (the Regulator) was not required to consult with prescribed experts, agencies Chapter 1 – Risk assessment context 1 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator and authorities before preparation of the Risk Assessment and Risk Management Plan (RARMP; see section 50 of the Act). Section 52 of the Act requires the Regulator to seek comment on the RARMP from the States and Territories, the Gene Technology Technical Advisory Committee, Commonwealth authorities or agencies prescribed in the Regulations, the Minister for the Environment, relevant local council(s), and the public. The advice from the prescribed experts, agencies and authorities and how it was taken into account is summarised in Appendix A. Five public submissions were received and their considerations are summarised in Appendix B. The Risk Analysis Framework (OGTR 2013) explains the Regulator’s approach to the preparation of RARMPs in accordance with the legislative requirements. Additionally, there are a number of operational policies and guidelines developed by the Office of the Gene Technology Regulator (OGTR) that are relevant to DIR licences. These documents are available from the OGTR website. Any dealings conducted under a licence issued by the Regulator may also be subject to regulation by other Australian government agencies that regulate GMOs or GM products, including Food Standards Australia New Zealand (FSANZ), Australian Pesticides and Veterinary Medicines Authority (APVMA), Therapeutic Goods Administration (TGA), National Industrial Chemicals Notification and Assessment Scheme and the Department of Agriculture. These dealings may also be subject to the operation of State legislation declaring areas to be GM, GM free, or both, for marketing purposes. Section 3 The proposed dealings Murdoch University proposes to release up to 35 lines2 of genetically modified (GM) wheat into the environment under limited and controlled conditions. The purpose of the trial is to assess whether the introduction and expression of the genes will increase the strength of bread dough. Published research has shown that increasing the levels of certain proteins by genetic transformation (ie introducing additional copies of the genes in order to increase the quantity of their corresponding proteins) is a useful way of generating novel doughs for characterisation. In addition, the release will allow the applicant to produce sufficient grain for subsequent replicated trials. The dealings involved in the proposed intentional release include: conducting experiments with the GMOs breeding the GMOs propagating the GMOs growing or culturing the GMOs transporting the GMOs disposing of the GMOs possession, supply or use of the GMOs for any of the purposes above. These dealings are detailed further below. 3.1 The proposed limits of the dealings (size, location, duration and people) The applicant proposes to grow GM wheat plants between May 2015 and December 2017. The term ‘line’ is used to denote plants derived from a single plant containing a specific genetic modification resulting from a single transformation event. 2 Chapter 1 – Risk assessment context 2 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator The GMOs are proposed to be planted in the New Genes for New Environment (NGNE) facility located at Katanning in Western Australia. This facility is operated by the Department of Agriculture and Food, Western Australia (DAFWA). The maximum area of the trial in any year would be up to 0.06 hectares (ha). Only trained and authorised staff are proposed to be permitted to deal with the GM wheat. Any other visitors to the trial site would be accompanied by an authorised Murdoch University representative and would not deal with the GMOs. 3.2 The proposed controls to restrict the spread and persistence of the GMOs and their genetic material in the environment The applicant has proposed a number of controls to restrict the spread and persistence of the GM wheat lines and the introduced genetic material in the environment, including: locating the NGNE facility at least 50 m from the nearest waterway surrounding the facility by a 1.8 m fence to exclude large animals and using a bird proof netting (these are existing features of the NGNE facility) implementing a rodent control program surrounding the facility by a 10 m monitoring zone and 190 m isolation zone in which no sexually compatible plants will be grown cleaning any equipment used with the GM plants before removal from the site, and the disposal of any material collected during cleaning in a manner approved by the Regulator separating the GM wheat plants by a buffer of at least 4 m if other GM wheat DIRs are being grown side by side in the same facility monitoring the planted locations at least once every fortnight during the flowering of the GM plants post-harvest monitoring of the trial site (once every 35 days) and destruction of any volunteer wheat for at least 24 months destroying any plant material collected during cleaning by autoclaving, hammer-milling, incineration, burial, or any other method approved by the Regulator ploughing back waste material and stubble from harvesting into the soil grain/dough testing in the PC2 laboratory at Katanning, all laboratory equipment being subjected to cleaning both before and after use transporting and storing GM material in accordance with the Regulator's Guidelines for the Transport, Storage and Disposal of GMOs (2011) not allowing GM plant material or products to be used for human food or animal feed. Figure 2 shows the proposed site layout at the Katanning NGNE facility, including some of these controls. These controls, and the limits outlined above, have been taken into account in establishing the risk assessment context (this Chapter), and their suitability for containing the proposed release is evaluated in Chapter 3, Section 3.1.1. Chapter 1 – Risk assessment context 3 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator 190 m wide Isolation Zone, inspected for wheat during the flowering of GMOs 2 m wide Buffer Zone where the growth of plants is controlled 10 m wide Monitoring Zone, where the growth of plants is controlled NGNE site Fence Planting area where GM wheat is planted Figure 2 Proposed trial layout, including some of the controls (not drawn to scale) Section 4 The parent organism The parent organism is bread wheat (Triticum aestivum L.), which is exotic to Australia. Commercial wheat cultivation occurs in the wheat belt from south eastern Queensland through New South Wales, Victoria, Tasmania, southern South Australia and southern Western Australia. All lines were initially in the cultivar Bobwhite, but were backcrossed into the cultivars Calingiri, Wyalkatchem, Westonia, and IGW2836 (NLRD 2341/2007). Detailed information about the parent organism is contained in the reference document The Biology of Triticum aestivum L. em Thell (bread wheat) (OGTR 2008), which was produced to inform the risk assessment process for licence applications involving GM wheat plants. This document is available from the OGTR website. Section 5 The GMOs, nature and effect of the genetic modification 5.1 Introduction to the GMOs The applicant proposes to release up to 35 lines of GM wheat. The lines were originally produced in the wheat cultivar Bobwhite by biolistics mediated plant transformation. Information about this transformation method can be found in the document Methods of plant genetic modification available from the Risk Assessment References page on the OGTR website. Each GM wheat line has been transformed with a construct containing one of two genes of interest, or a hybrid of these genes. The GM plants also contain the selectable marker gene bar, originating from Streptomyces hygroscopicus. The expression of the individual Dx5 and Dy10 genes in the GM wheat lines are controlled by their native 5’ and 3’ regulatory sequences. For the Dy10-Dx5 hybrid gene, the expression is controlled by the Dy10 promoter and the Dx5 terminator. The bar gene is driven by the promoter consisting of the 5’ untranslated exon and first intron of the maize ubiquitin (ubi-1) gene (Christensen & Quail 1996; Christensen et al. 1992), and followed by the nos terminator from Agrobacterium tumefaciens. A summary of these genes is presented in Table 1. Chapter 1 – Risk assessment context 4 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Table 1 Summary of genes and regulatory elements introduced into the GM wheat plants. Promoter (origin) Dx5 (wheat) Dy10 (wheat) Dy10 (wheat) Ubi-1 (maize) Gene of interest (origin) Dx5 (wheat) Dy10 (wheat) Dy10-Dx5 hybrid (Sequences of wheat genes Dx5 and Dy10) bar (Streptomyces hygroscopicus) Terminator (origin ) Dx5 (wheat) Dy10 (wheat) Dx5 (wheat) nos (Agrobacterium tumefaciens) 5.2 The introduced genes, encoded proteins and their associated effects 5.2.1 Glutenin genes The application involves a field trial of GM wheat plants containing either of two wheat glutenin genes, Dx5 and Dy10 (Anderson & Greene 1989; Anderson et al. 1989), or a hybrid gene consisting of a single open reading frame of sequences derived from both Dx5 and Dy10 (Blechl & Anderson 1996). The hybrid gene codes for a mature protein that consists of amino acids 1-124 from Dy10 fused N-terminal to amino acids 130-848 from Dx5 (Blechl & Anderson 1996). Glutenins are seed storage proteins that act in germinating seeds as a supply of elements such as carbon, nitrogen and sulphur. They are divided into two classes: low molecular weight glutenin subunits (LMW-GSs) and high molecular weight glutenin subunits (HMW-GSs) (Akagawa et al. 2007; Battais et al. 2008; Wieser 2007). The production of wheat dough is dependent upon the structure and composition of the glutenin and gliadin seed storage proteins, which in the presence of water crosslink to form a matrix, gluten. In particular, the HMW-GSs, such as Dx5 and Dy10, play a critical role in determining the viscoelastic properties that are crucial for the formation of bread dough (Shewry et al. 2003). Bread wheat has three loci, designated Glu-A1, Glu-B1 and Glu-D1, that contain HMWGS genes, these being located on the long arms of chromosomes 1A, 1B and 1D, respectively (Shewry et al. 2003; Shewry & Tatham 1997). Each locus contains two genes, one gene encoding an x-type and the other a y-type subunit. The amino acid sequences of these subunits are characterised by highly repeated blocks of sequence. Both x -type and y -type subunits contain hexapeptide and nonapeptide motifs, the major difference between these two types of subunits being that the former also contains tripeptide motifs (Shewry et al. 1992; Shewry et al. 2003). Each gene has alleles. As specfic x-type and y-type alleles usually occur together at a locus, their separation by recombination is difficult; this gene pair hence constitute what can be regarded as a single allele. The Dx5 and Dy10 genes occur together as an ‘allele’ (Glu-D1d) of the locus located on chromosome 1D. Other ‘alleles' at this locus include Dx2+Dy12 (Glu-D1a) and Dx4+Dy12 (Glu-D1b) (Zheng et al. 2011). In many plants, certain HMW-GS genes are expressed at low levels or silent (Forde et al. 1985; Wieser & Zimmermann 2000). As such, a plant commonly produces a smaller number of HMW-GS proteins than may be theoretically predicted (Anjum et al. 2007; Shewry et al. 2003). The properties of dough that are linked to glutenin have been investigated by a number of studies that have involved the transformation into wheat of glutenin genes (Blechl et al. 2007). Many of these experiments have involved the co-bombardment of immature embryos with high copy number plasmids containing the genes of interest and a plasmid with a herbicide tolerant gene to enable selection of transformants. In one study, aimed at evaluating agronomic performance of bread wheat lines transformed with glutenin genes, the introduction of Dx5 and Dy10 did not show significant changes in traits associated with performance (Bregitzer et al. 2006). Other research with varieties of bread wheat transformed with the Dx5 and Dy10 genes has shown that both these genes affect the mixing properties of dough, but in different ways (Blechl et al. 2007). Increasing the level of the Dy10 protein over five times above that normally found in wheat plants did not prevent dough mixing. However, smaller increases in the Dx5 protein led to greater dough strength, problems with mixing, higher amounts of polymeric protein and lower sedimentation values. It has Chapter 1 – Risk assessment context 5 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator been suggested that these differences between these two HMW-GSs is possibly due to variations between the sizes and composition of their repetitive domains, and their abilities to form both intermolecular and intramolecular disulphide bonds (Blechl et al. 2007). The studies described above were conducted in a wheat background containing endogenous Dx5 and Dy10 genes. Other work has involved separately transforming the Dx5 and Dy10 genes into a genetic background that does not contain these genes, thus enabling the individual effects of these two genes on bread quality to be more accurately evaluated (Leon et al. 2009). Expression of Dy10 alone resulted in lines with greater dough strength than those expressing Dx5. Conventional crossing of these lines demonstrated that a combination of Dx5 and Dy10 produced a dough with the greater strength and better dough mixing properties than some other subunit combinations that were evaluated (Leon et al. 2010). Varieties of durum wheat (Triticum turgidum L. ssp. durum) have also been co-transformed with the wheat genes Dx5 and Dy10 (Gadaleta et al. 2008). The mixing times and peak resistances of dough from the transformed lines were likewise greater than those recorded in the wild-type parents, indicating that expression of the inserted genes was having a positive effect upon strength. With respect to the wild-type parent varieties, yields from the transformed lines were similar. In another line of research, durum wheat varieties separately expressing the HMW-GS 1Ax1 gene and the Pina gene linked to grain hardness were generated, and then conventional breeding used to combine the two genes (Li et al. 2010). Analysis of these lines suggested that overexpression of both these genes can enhance dough strength. The effects of changing the levels of gliadins on dough properties has also been investigated, the down-regulation of these genes being mediated by RNAi (Gil-Humanes et al. 2012; Gil-Humanes et al. 2008). Examination of the protein complement of wheat plants that have been transformed via biolistics with HMW-GS genes has revealed that there are frequently HMW-GS proteins that are either larger or smaller than the predicted sizes (Blechl & Vensel 2013). These are likely the result of transformation events that alter the sequence of the genes or result in tandem repeats. 5.2.2 Selectable marker genes All the GM wheat lines contain the selectable marker gene bar, derived from Streptomyces hygroscopicus. This gene codes for the enzyme phosphinothricin N-acetyltransferase (PAT), which provides tolerance to the broad spectrum herbicide phosphinothricin, also known as glufosinate ammonium. This gene has been used extensively as a selectable marker gene in the production of GM plants. More information on selectable marker genes in general, can be obtained from the OGTR document Marker genes in GM plants, available on the OGTR website. The plants may also contain the bacterial ampicillin resistance (bla) gene (Sutcliffe 1979), which codes for the enzyme beta-lactamase. The promoter of this gene is specific for prokaryotic organisms; thus the ampicillin protein will not be expressed in plants. 5.3 Toxicity/allergenicity or other adverse effects upon health associated with the introduced genes, their encoded proteins and associated products The introduced genes of interest, Dx5 and Dy10, originate from wheat. This plant is widely consumed by people and animals, and as such people and animals have a long history of exposure to its proteins. There is no evidence that wheat has any toxic properties. However, a minority of people suffer from allergies to wheat (and other cereals). The two most well characterized allergies to wheat are baker’s asthma (induced by the inhalation of wheat flour during grain processing) and exercise induced anaphylaxis (a reaction that occurs if someone undergoes physical activity soon after consumption of wheat) (Hischenhuber et al. 2006; Tatham & Shewry 2008). These allergies are likely due to a number of compounds in cereals, although the most important triggers are believed to be the -amylase inhibitors and gluten (glutenin and gliadin) seed storage proteins (Battais et al. Chapter 1 – Risk assessment context 6 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator 2008; Salcedo et al. 2011; Tatham & Shewry 2008). Rashes, wheezing, swelling and abdominal pains are all symptoms of wheat allergy, while pathogenic mechanisms of induction include IgEmediated and cell-mediated (Hischenhuber et al. 2006). Wheat allergy is often a temporary condition of young children that is outgrown by the age of five (Pietzak 2012). Both HMW-GSs and LMW-GSs have been implicated in wheat allergy. Experiments have suggested that HMW-GS proteins are allergens in exercise induced anaphylaxis (Matsuo et al. 2005; Morita et al. 2009; Yokooji et al. 2013). Recombinant expressed proteins have been shown to react with sera from patients with wheat allergies (Eriksson et al. 2012; Maruyama et al. 1998). Screening of a wheat cDNA expression library with antisera from wheat allergenic patients identified clones coding for HMW-GS, three likely representing a Dx gene and three Bx7, other experiments identifying the latter as an important allergen (Baar et al. 2014). Three specific sequences in HMW-GSs (which occur in both the Dx5 and Dy10 proteins (Anderson et al. 1989; Sugiyama et al. 1985; Thompson et al. 1985)) have been identified as IgE-binding epitopes (Matsuo et al. 2005). In the case of LMW-GS proteins, a proteomic study of wheat allergens identified nine members of this class as important allergens, but could not confirm previously reported examples classified as HMW-GS (Akagawa et al. 2007). Techniques such as screening of a wheat cDNA library with sera from patients with wheat allergy have also suggested a connection of LMW-GS proteins to this condition (Baar et al. 2012). Coeliac (celiac) disease is an autoimmune (genetic) disorder that is triggered by the consumption of gluten (Denham & Hill 2013; Hischenhuber et al. 2006). It is characterised by the immune system attacking the small intestine and inhibiting the absorption of nutrients into the body, likely leading to permanent tissue damage. People can also suffer from gluten intolerance (gluten sensitivity), an adverse reaction to wheat that is neither an allergenic reaction or mediated by the immune system (Pietzak 2012; Sapone et al. 2012). Distinct varieties of wheat often have different quantities (per grain) of glutenin and gliadin proteins, the quantities in any variety also being influenced by the environment in which the plant is grown (Plessis et al. 2013). From a molecular viewpoint, the synthesis of these proteins is likely regulated primarily at the transcriptional level. Activation and down-regulation of the expression of their respective genes probably involves a number of different transcription factors binding to each other, and either directly or indirectly to DNA promoter elements. Transcription factors that have been associated with gluten protein levels include members of the Opaque2 subfamily of basic leucine zipper (bZIP) factors (storage protein activators) (Albani et al. 1997; Ravel et al. 2009) and DOF (DNA binding with one finger) proteins (Dong et al. 2007; Romeuf et al. 2010). The levels of such transcription factors, and hence seed storage proteins, may be linked to biochemical issues, including the assimilation of nitrogen and the availability of amino acids. Quantitative trait loci linked to the levels of gluten proteins have also been identified and mapped (Zhang et al. 2011). 5.4 Characterisation of the GMOs 5.4.1 Stability and molecular characterisation Based on experiments in the United States, the genes are stably inserted into the genome of the cultivar Bobwhite. There is no reason to expect that conventionally breeding of these genes into other cultivars will affect their stability. However, the sites of insertion and copy numbers of the inserted genes are not known. The proposed field trial will evaluate the expression of the genes in a number of Australian cultivars. 5.4.2 Phenotypic characterisation Preliminary phenotypic characterisation of the plants in glasshouses has demonstrated that the introduced genes induce no major visible phenotypes or reduce viability. Chapter 1 – Risk assessment context 7 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Section 6 The receiving environment The receiving environment includes: any relevant biotic/abiotic properties of the geographic regions where the release would occur; intended agricultural practices, including those that may be altered in relation to normal practices; other relevant GMOs already released; and any particularly vulnerable or susceptible entities that may be specifically affected by the proposed release (OGTR 2013). The factors relevant to the growth, distribution and cultivation of commercial wheat can be found in The Biology of Triticum aestivum L. em Thell (Bread Wheat) (OGTR 2008). It is proposed that the dealings be conducted in the NGNE facility, operated by DAFWA, located at Katanning in Western Australia. 6.1 Relevant abiotic factors The Katanning NGNE facility is purpose built for the trialling of GM plants. Katanning represents the high rainfall environment used for growing wheat in Western Australia. The soil of Katanning is largely alkaline and sodic. 6.2 Relevant agricultural practices It is not anticipated that the agronomic practices for the cultivation of the GM wheat by the applicant will be significantly different from conventional practices for these plants. GM wheat seeds would be planted in the trial site in winter or early spring. Seed that remain after harvest would be either stored in an approved facility for subsequent use or destroyed. Volunteers would be removed by hand or killed by herbicide application. 6.3 Presence of related plants in the receiving environment Wheat (Triticum aestivum L.) is sexually compatible with a number of species within the tribe Triticeae that occur in Australia. Of particular importance are durum wheat (Triticum turgidum ssp. Durum), rye (Secale cereale), and Triticale. Hybridisation with durum wheat occurs readily (Wang et al. 2005), whereas that with rye (Dorofeev 1969; Leighty & Sando 1928; Meister 1921) and Triticale (Ammar et al. 2004; Kavanagh et al. 2010) is rarer. Wheat also readily hybridises with Aegilops species, but no Aegilops species are considered to be naturalised in Australia. Any specimens of Aegilops that have been collected in Australia presumably originate from seed accidently introduced amongst wheat seed, or straying from that brought in for breeding programs (AVH 2012). Australasia possesses four native Triticeae genera - Australopyrum, Stenostachys, Anthosachne (Elymus), and Connorochloa (Barkworth & Jacobs 2011) – as well as a number of introduced species of Triticeae, such as Elytrigia repens (couch grass) and at least four Thinopyrum species (Bell et al. 2010). Thinopyrum ponticum (tall wheatgrass) has been used as a saltland pasture plant in Australia, and in some regions has come to be classified as a weed (Barrett-Lennard 2003; NYNRMP 2011). Although there has been no concerted investigation of natural hybridisation of these native and introduced Triticeae species with wheat, based on experience of hybridising wheat with most other members of the Triticeae, it is unlikely that it occurs. As the Katanning NGNE facility is designed to be accommodate more than one trial (which may reflect more than one licence holder), it is possible that other GM and non-GM wheat plants will be grown there in close proximity to those plants that are the subject of this application. Currently, GM wheat plants from DIR 128 which are genetically modified for abiotic stress tolerance or micronutrient uptake may be present. 6.4 Presence of similar genes and encoded proteins in the environment The introduced genes and other genetic elements are from a plant (wheat) that is widespread and prevalent in the environment (see Section 5). It is commonly consumed by people who are Chapter 1 – Risk assessment context 8 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator therefore exposed to the proteins. Other cereals (that are consumed by people) have glutenin proteins (Shewry & Halford 2002). The bar selectable marker gene comes from Streptomyces hygroscopicus, which is widespread in the environment. Section 7 Relevant Australian and international approvals 7.1 Australian approvals 7.1.1 Approval by the Regulator Wheat lines possessing the introduced genes have not previously been approved by the Regulator. Information on previous DIR licences for GM wheat can be found on the GMO Record on the OGTR website. 7.1.2 Approval by other government agencies No other approvals are currently required for this GM wheat trial. 7.2 International approvals of GM wheat Field trials of the GM wheat lines in the Bobwhite genetic background have been carried out in the USA in 2002 and 2003 (information provided by the applicant). Field trials of different GM wheat plants have been approved internationally, including in the USA, Canada, Germany, Czech Republic, Denmark, Hungary, Iceland, Italy, Spain, Sweden and the United Kingdom. The traits that have been modified include: novel protein production, disease resistance, insect resistance, altered grain properties and herbicide tolerance3. 3 USDA release permit applications, EU GMO Register, accessed November 2014. Chapter 1 – Risk assessment context 9 DIR 130 – Risk Assessment and Risk Management Plan Chapter 2 Office of the Gene Technology Regulator Risk assessment Section 1 Introduction The risk assessment identifies and characterises risks to the health and safety of people or to the environment from dealings with GMOs, posed by or as the result of gene technology (Figure 3). Risks are identified within the context established for the risk assessment (see Chapter 1), taking into account current scientific and technical knowledge. A consideration of uncertainty, in particular knowledge gaps, occurs throughout the risk assessment process. RISK ASSESSMENT PROCESS * Postulation of risk scenarios Risk context Identification of substantive risks Consequence assessment Substantive Risks Risk scenarios Risk Evaluation Likelihood assessment Negligible risks RISK IDENTIFICATION RISK CHARACTERISATION * Risk assessment terms are defined in the Risk Analysis Framework 2013 Figure 3 The risk assessment process Initially, risk identification considers a wide range of circumstances whereby the GMO, or the introduced genetic material, could come into contact with people or the environment. Consideration of these circumstances leads to postulating plausible causal or exposure pathways that may give rise to harm for people or the environment from dealings with a GMO (risk scenarios) in the short and long term. Postulated risk scenarios are screened to identify substantive risks that warrant detailed characterisation. A substantive risk is only identified for further assessment when a risk scenario is considered to have some reasonable chance of causing harm. Pathways that do not lead to harm, or could not plausibly occur, do not advance in the risk assessment process. A number of risk identification techniques are used by the Regulator and staff of the OGTR, including checklists, brainstorming, reported international experience and consultation (OGTR 2013). A weed risk assessment approach is used to identify traits that may contribute to risks from GM plants. In particular, novel traits that may increase the potential of the GMO to spread and persist in the environment or increase the level of potential harm compared with the parental plant(s) are used to postulate risk scenarios (Keese et al. 2013). In addition, risk scenarios postulated in previous RARMPs prepared for licence applications of the same and similar GMOs are also considered. Chapter 2 – Risk assessment 10 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Substantive risks (i.e. those identified for further assessment) are characterised in terms of the potential seriousness of harm (Consequence assessment) and the likelihood of harm (Likelihood assessment). Risk evaluation then combines the Consequence and Likelihood assessments to determine level of risk and whether risk treatment measures are required. The potential for interactions between risks is also considered. Section 2 Risk Identification Postulated risk scenarios are comprised of three components (Figure 4): i. The source of potential harm (risk source). ii. A plausible causal linkage to potential harm (causal pathway). iii. Potential harm to an object of value, people or the environment. source of potential harm potential harm to plausible causal linkage (a novel GM trait) an object of value (people/environment) Figure 4 Risk scenario In addition, the following factors are taken into account when postulating relevant risk scenarios: the proposed dealings, which may be to conduct experiments, develop, produce, breed, propagate, grow, import, transport or dispose of the GMOs, use the GMOs in the course of manufacture of a thing that is not the GMO, and the possession, supply and use of the GMOs in the course of any of these dealings the proposed limits including the extent and scale of the proposed dealings the proposed controls to limit the spread and persistence of the GMO characteristics of the parent organism(s). 2.1 Risk source The source of potential harms can be intended novel GM traits associated with one or more introduced genetic elements, or unintended effects/traits arising from the use of gene technology. As discussed in Chapter 1, each of the GM wheat lines has been modified by the introduction of one of two HMW-GS genes, or a hybrid of both, that are expected to confer improvement in grain quality. These introduced genes are considered further as potential sources of risk. The GM lines also contain the bar selection marker gene (see Chapter 1, Section5.2.2). This gene and its product has already been extensively characterised and assessed as posing negligible risk to human or animal health or to the environment by the Regulator as well as other regulatory agencies in Australia and overseas. Toxicity feeding study expermiments have failed to establish any deleterious effects of the PAT protein upon animals (Herouet et al. 2005; MacKenzie et al. 2007; Merriman 1996). More information on selectable marker genes can be obtained from the OGTR document Marker genes in GM plants, available on the OGTR website. As this gene has not been found to pose substantive risks to either people or the environment, its potential effects will not be further assessed for this application. Chapter 2 – Risk assessment 11 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator In addition, the GM plants may also contain the bla gene (see Chapter 1, Section5.2.2). There is no evidence that the protein encoded by this gene has toxic or allergenic properties. The RARMPs for DIRs 070/2006 and 071/2006 concluded that the risks posed from the expression of a prokaryotic promoter driven bla gene in GM plants were negligible. Even if the bla gene was expressed in the wheat, human exposure to the beta-lactamase protein is routine as ampicillin resistant bacteria occur widely in the environment. For example, E.coli with such a trait is common in the normal human intestine (EFSA 2004; EFSA 2009). Therefore, this gene will not be assessed further for this application. The HMW-GS genes include endogenous wheat regulatory sequences while the promoter of the bar gene is derived from a maize ubiquitin gene. There is no evidence that regulatory sequences themselves have toxic or allergenic effects (EPA 1996); such effects for these sequences will not be further assessed for this application. However, regulatory sequences, especially the promoters, control the levels of gene expression and hence the levels of the derived proteins in the GM plants. The effects of these protein levels on, in particular, the toxicity and allergenicity of these plants (or at least materials derived from them), will be discussed below. 2.2 Causal pathway The following factors are taken into account when postulating plausible causal pathways to potential harm: routes of exposure to the GMOs, the introduced gene(s) and gene product(s) potential effects of the introduced gene(s) and gene product(s) on the properties of the organism potential exposure to the introduced gene(s) and gene product(s) from other sources in the environment the environment at the site(s) of release agronomic management practices for the GMOs spread and persistence (invasiveness) of the GM plant, including o establishment o reproduction o dispersal by natural means and by people tolerance to abiotic conditions (eg climate, soil and rainfall patterns) tolerance to biotic stressors (eg pest, pathogens and weeds) tolerance to cultivation management practices gene transfer to sexually compatible organisms gene transfer by horizontal gene transfer (HGT) unauthorised activities. Although all of these factors are taken into account, some have been considered in previous RARMPs or are not expected to give rise to substantive risks. The potential for horizontal gene transfer (HGT) and any possible adverse outcomes has been reviewed in the literature (Keese 2008) as well as assessed in many previous RARMPs. HGT was most recently considered in the RARMP for DIR 108. This and other RARMPs are available from the GMO Record on the OGTR website or by contacting the OGTR. No risk greater than negligible was identified due to the rarity of these events and because the wild-type gene sequences are already present in the environment and available for transfer via demonstrated natural mechanisms. Therefore, HGT will not be assessed further. Chapter 2 – Risk assessment 12 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator The potential for unauthorised activities to lead to an adverse outcome has been considered in previous RARMPs. The Act provides for substantial penalties for non-compliance and unauthorised dealings with GMOs. The Act also requires the Regulator to have regard to the suitability of the applicant to hold a licence prior to the issuing of a licence. These legislative provisions are considered sufficient to minimise risks from unauthorised activities, and no risk greater than negligible was identified in previous RARMPs. Therefore, unauthorised activities will not be considered further. 2.3 Potential harm Potential harms from GM plants include: harm to the health of people or desirable organisms, including toxicity/allergenicity reduced establishment of desirable plants, including having an advantage in comparison to related plants reduced yield of desirable vegetation reduced products or services from the land use restricted movement of people, animals, vehicles, machinery and/or water reduced quality of the biotic environment (eg providing food or shelter for pests or pathogens) or abiotic environment (eg negative effects on fire regimes, nutrient levels, soil salinity, soil stability or soil water table) reduced biodiversity through harm to other organisms or ecosystems. These harms are based on those used to assess risk from weeds (Standards Australia 2006). Judgements of what is considered harm depend on the management objectives of the land where the GM plant is expected to spread to and persist. A plant species may have different weed risk potential in different land uses such as dryland cropping or nature conservation. 2.4 Postulated risk scenarios Five risk scenarios were postulated and screened to identifiy substantive risk. These scenarios are summarised in Table 2 and more detail of these scenarios is provided later in this Section. Postulation of risk scenarios considers impacts of the GM wheat or their products on people undertaking the dealings, as well as impacts on people and the environment if the GM plants or genetic material were to spread and/or persist. In the context of the activities proposed by the applicant and considering both the short and long term, none of the five risk scenarios gave rise to any substantive risks. Table 2 Summary of risk scenarios from dealings with GM wheat genetically modified for improved grain quality Risk Risk source scenario 1 Introduced genes for improved grain quality Chapter 2 – Risk assessment Causal pathway Potential harm Growing GM plants at the site Expression of genes in GM plants Exposure of people who specifically deal with the GM plant material or other organisms that come into contact with the GM plant material in the trial site Allergic reactions in people or toxicity in people and other organisms Substantive risk? No Reason The limited scale, short duration and other proposed limits and controls minimise exposure of people and other organisms to the GM plant material. Plant material from the GMOs would not be used for human food or animal feed. 13 DIR 130 – Risk Assessment and Risk Management Plan Risk Risk source scenario Causal pathway Office of the Gene Technology Regulator Potential harm Substantive risk? 2 Introduced genes for improved grain quality Dispersal of GM seed outside trial limits Growth of GM plants Expression of genes in GM plants Spread and persistence of populations of GM plants outside trial limits Exposure of people or other organisms to GM plant material Allergic reactions in people or toxicity in people and other organisms Reduced establishment and yield of desirable plants Reduced biodiversity No 3 Introduced genes for improved grain quality Dispersal of GM pollen outside trial limits Vertical transfer of introduced genes to other sexually compatible plants, such as commercial varieties of wheat Expression of genes in plants Exposure of people or other organisms to GM plant material Allergic reactions in people or toxicity in people and other organisms Reduced establishment and yield of desirable plants Reduced biodiversity No 4 Introduced genes for improved grain quality Dispersal of GM pollen within the NGNE facility Hybridisation of GM plants of this trial with GM plants (including volunteers) of another trial Expression of genes in stacked GM plants Exposure of people or other organisms to GM plant material Allergic reactions in people or toxicity in people and other organisms No Chapter 2 – Risk assessment Reason The introduced proteins are not known to be toxic. Although they have been associated with allergenicity, there is uncertainty whether they will affect the allergenic threshold in individuals. The limited scale, short duration and other proposed limits and controls minimise the likelihood that GM plant material would leave a trial site. The introduced proteins are not known to be toxic. Although they have been associated with allergenicity, there is uncertainty whether they will affect the allergenic threshold in individuals. The introduced genes are not expected to increase the ability of the GM plants to spread and persist. The limited scale, short duration and other proposed limits and controls minimise the likelihood that GM plant material would leave a trial site. The introduced proteins are not known to be toxic. Although they have been associated with allergenicity, there is uncertainty whether they will affect the allergenic threshold in individuals. The introduced genes are not expected to increase the ability of the GM plants to spread and persist. The limited scale, short duration and other proposed limits and controls minimise exposure of people and other organisms to the GM plant material. The introduced proteins are not known to be toxic. Although they have been associated with allergenicity, there is uncertainty whether they will affect the allergenic 14 DIR 130 – Risk Assessment and Risk Management Plan Risk Risk source scenario 5 2.4.1 Introduced genes for improved grain quality Office of the Gene Technology Regulator Causal pathway Potential harm Dispersal of GM pollen within NGNE site Hybridisation of GM plants of this trial with GM plants (including volunteers) of another trial Dispersal of plants or viable plant material containing stacked genes outside the trial limits Expression of genes in stacked GM plants Spread and persistence of populations of GM plants outside a trial site Reduced establishment and yield of desirable plants Reduced biodiversity Substantive risk? No Reason threshold in individuals. The stacking of genes from different GM plants is unlikely to increase the toxicity or allergenicity of the hybrid GM plants. The limited scale, short duration and other proposed limits and controls minimise the likelihood that GM plant material would leave a trial site. The introduced genes are not expected to increase the ability of the GM plants to spread and persist. The stacking of genes from different GM plants is unlikely to increase the weediness of the hybrid GM plants. Risk scenario 1 Risk source Introduced genes for improved grain quality Causal pathway Growing GM plants at the site Expression of genes in GM plants Exposure of people who specifically deal with the GM plant material or other organisms that come into contact with the GM plant material in the trial site Potential harm Allergic reactions in people or toxicity in people and other organisms Risk source The source of potential harm for this postulated risk scenario is the introduced genes for improved grain quality. Causal pathway The grain quality improvement genes are expressed in the plant tissues. People who are involved in the breeding, cultivating, harvesting, transporting and experimenting of the GM wheat may be exposed to its products through contact (including inhalation of pollen). This would be expected to mainly occur in the trial site, but could also occur anywhere the GM plant material was transported or used for experimental analysis. Organisms that may be present in the trial site, including birds, rodents and invertebrates, may be exposed to the GM plant material. The proposed limits and controls of the trial would minimise the likelihood that people or other organisms would be exposed to GM plant material. Although people may directly handle the GM plant material, it is not to be used as human food. Further, as the trial is limited to a single site Chapter 2 – Risk assessment 15 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator of 0.06 ha, only a small number of people would deal with the GM plant material and a small number of other organisms are likely to be exposed to it. GM plant material is not to be used as animal feed. A fence surrounding the NGNE facility will exclude livestock and other large animals, while rodent control measures will be used to reduce the number of these animals. Further, the facility has bird netting. The applicant has also proposed a series of measures, such as the monitoring and inspecting of the site, together with the cleaning of equipment, the storing of seed and the disposal of waste material that will together help reduce exposure of people to GM plant material in the trial site and the adjacent areas. Potential harm People exposed to the proteins expressed from the introduced genes or their associated products may show toxic or allergic reactions, while other organisms may show toxic reactions (Chapter 2, Section 2.3). Proteins are not generally associated with toxic effects. As opposed to small molecular weight chemicals (eg pesticides), proteins have a number of properties that limit their ability to produce toxic effects upon ingestion, these including their likely digestion in the gastrointestinal tract and difficulties they encounter in traversing plasma membranes (Hammond et al. 2013). However, a small number of proteins have been shown to be toxic to humans and mammals, these originating mainly from animals (eg snakes, scorpions) and bacteria (Henkel et al. 2010; Karalliedde 1995). Lectins and protease inhibitors are plant proteins that have toxic properties, but for most people the level of exposure and response to the majority of these compounds is such that they are often classified as anti-nutrients (Delaney et al. 2008). The most well known plant proteins that are definitely toxic to humans are the lectins that consist of a ribosome-inactivating peptide (RIP) linked to a carbohydrate binding peptide, examples being ricin, abrin and modeccin (de Virgilio et al. 2010; Stirpe 2005; Wu & Sun 2011). Other plant proteins, found to be toxic at least to mice, include the urease-like-protein canatoxin from jack beans (Follmer et al. 2001) and an acidic protein and a glycoprotein from soybean (Morais et al. 2010; Vasconcelos et al. 2008; Vasconcelos et al. 1994). All known food allergens are proteins, those derived from plants coming chiefly from peanut, tree nuts, wheat and soybean (Delaney et al. 2008; Herman & Ladics 2011). The structural and functional properties of plant food allergens can be used to classify them into approximately 30 families, these then being grouped into a small number of superfamilies (Hauser et al. 2008; Radauer & Breiteneder 2007; Salcedo et al. 2008). The major superfamilies are the prolamins, cupins, pathogenesis-related (PR) proteins, profilins and protease inhibitors. Beyond toxicity and allergenicity, specific proteins and chemicals have been associated with autoimmune diseases and food intolerances (ASCIA 2014; Barragan-Martinez et al. 2012; Selmi et al. 2012). Chapter 1, Section 5.3 presents a review of the potential toxic and allergenic properties of the proteins encoded by the introduced genes. It was concluded that none of the introduced proteins were likely to be toxic to people or other organisms. In respect of the general information on toxicity and plant proteins outlined above, none of the introduced plant proteins can be classified as a lectin or protease inhibitor (ie a likely toxin). The glutens are prominent members of the prolamin family that have been associated with allergenicity, and, as noted in Chapter 1, Section 5.3, the HMW-GS proteins have been associated with negative effects on the health of people. These effects have been grouped as allergies (eg the consumption of glutenin can induce baker’s asthma or exercise induced anaphylaxis), autoimmune diseases (eg coeliac disease) and ‘gluten sensitivity’ (Sapone et al. 2012). Specific studies that have used the Dx5 and Dy10 proteins, or epitope peptides based on their sequences, have implicated these HMW-GSs in exercise induced anaphylaxis (Matsuo et al. 2005; Morita et al. 2009; Yokooji et al. 2013). Chapter 2 – Risk assessment 16 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator For the evaluation of protein safety, the ILSI International Food Biotechnology Committee has collaborated with a group of experts to produce a two tiered method to assess the safety of proteins (Delaney et al. 2008; Hammond et al. 2013). The first tier examines five issues: (i) history of safe use of the protein; (ii) bioinformatics analysis; (iii) mode of action; (iv) in vitro digestibility and stability; and (v) expression level and dietary intake. Only if potential safety issues were identified in this evaluation would a second tier assessment be recommended, a prime example of such an assessment being a dose toxicology study. The proteins Dx5 and Dy10 were examined against the first tier criteria: (i) History of safe use. The introduced plant proteins come from a plant (wheat) that does not raise any toxicological concerns and has a history of largely safe use in human diets. A minority of people have allergenic reactions to wheat, possess coeliac disease or have ‘gluten sensitivity’. (ii) Bioinformatic analysis. The introduced proteins do not have any sequence similarity with known toxins. However, they are members of a protein class, the prolamins, that is known to have members with allergenic properties. (iii) Mode of action. Plants are responsible for the production of a wide range of small molecular weight compounds, usually designated as secondary metabolites, many of which are toxic to humans and herbivore animals (Wink 2009; Wink & Van Wyk 2008). From the perspective of the plant, they help defend the plant against the predatory activities of these groups. The metabolites of greatest concern are the neurotoxins, followed by cytotoxins and compounds that act as poisons in particular organs. The introduced proteins are considered as storage proteins for amino acids that can be used in germination and seedling growth (Shewry & Halford 2002). They do not have enzymatic functions. As such, they are not expected to give rise to any (enzymatic) small molecular weight products that could have toxic properties. (iv) In vitro digestibility and stability. No data. (v) Expression level and dietary intake. No data. The hybrid gene codes for a protein that consists of the 124 N-terminal amino acids from the mature Dy10 protein fused N terminal to the C terminal 719 amino acids from Dx5 (Blechl & Anderson 1996). Such a protein does not have a history of safe use, but the experience of conventional breeding is that the spurious fusing of gene sequences does not lead to proteins that are of health or environmental concern (Steiner et al. 2013; Weber et al. 2012). Further, as discussed above, the sequences of Dx5 and Dy10 themselves have not been associated with toxicity, and it is not expected that a fusion protein of these sequences will have a mode of action that is different from its progenitors. Although the Dx5 and Dy10 proteins are endogenous to wheat, it is possible that increased quantities of one or both of these proteins may affect the allergenic threshold level of wheat with respect to gluten. The threshold level of a food can be defined as the maximum quantity of a food, containing one or more allergenic proteins, that can be tolerated without producing any adverse (allergenic) reaction. It can be measured by a number of methods, including epidemiological studies, analytical procedures and anecdotal evidence (FDA 2006). In these circumstances, data from points (iv) and (v) may take on greater importance. The threshold for eliciting an allergic reaction by a single allergen has rarely been determined, varies between individual people, and can vary in any one individual over time due to factors such as stress, exercise and the use of medications (Fernandez et al. 2013). Further, there have been few studies of the concentrations of endogenous food allergens across different cultivars of one species. When such studies have been conducted, allergen concentrations have been discovered to vary widely between cultivars, and a range of environmental factors can affect the level in any individual cultivar (Fernandez et al. 2013; Herman & Ladics 2011). No mechanisms are presently in place to Chapter 2 – Risk assessment 17 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator evaluate and segregate ‘low’ and ‘high’ allergenic cultivars of plants that constitute our food supply (Panda et al. 2013). With such a background, it is not possible to accurately answer the question of how much of an increase in an endogenous allergen would lead to a measurable increase in the level of harm. Nevertheless, it is possible that an elevation in the level of HMW-GSs in GM wheat could increase the spectrum of people who cannot tolerate food derived from this plant. Although people with food allergies largely avoid the offending foods altogether (gluten-free food being defined by standard 1.2.8, clause 16, of the Australia New Zealand Food Standards Code as ‘no detectable gluten’ (http://www.comlaw.gov.au/Details/F2012C00218)), there are individuals with moderate allergies who know, through experience, they can consume limited amounts of the these foods. The consequence to such individuals of an increase in allergen levels in GM food could be an elicitation of a severe allergenic response (Fernandez et al. 2013). However, as the GM wheat will not be used for human food, no harm as a result of humans consuming the GM wheat will occur. Experiments with the promoter of the Dx5 gene fused to the GUS reporter gene have demonstrated that this promoter drives expression of a gene in the endosperm of seeds, with no expression being detected in leaf, root inflorescence or floret tissues (Lamacchia et al. 2001). Similar results have been obtained when other HMW-GS promoters have been used to drive reporter genes (Furtado et al. 2009; Weibo et al. 2009). As glutenins are classified as seed storage proteins, these patterns of expression are not unexpected. Thus, in relation to the GM wheat of this application, there is unlikely to be a risk of a pollen-induced allergic reaction, but a possible route that people handling plant material may be exposed to the proteins is through contact with seed. Nevertheless, there is uncertainty as to whether the expression of Dx5 and Dy10 would affect the allergenicity of the GM wheat proposed for release. Gene technology has the potential to cause unintended effects in several ways, including altered expression of an endogenous gene by random insertion of an introduced DNA in the genome, increased metabolic burden due to higher expression of the introduced protein, novel traits arising out of interactions with non-target proteins and secondary effects arising from altered substrate or product levels in biochemical pathways. Such an effect could lead to elevation of the concentration of a normally benign wheat compound to a level where it induces a toxic or allergenic reaction if consumed in an average diet. It is also possible that an entirely novel compound could be produced with such a reaction. In this context, it is important to note that changes of this nature, such as the unexpected increase in the level of an endogenous toxin, can also be induced in plants by conventional methods of plant breeding (Haslberger 2003). However, even though conventional breeding can involve the movement of hundreds and even thousands of genes into a plant, there has never been a report of a completely novel toxin or allergen appearing in a new line of a plant produced by such techniques (Steiner et al. 2013; Weber et al. 2012). The implication is that the movement into wheat of any of the genes that are the subject of this application, none of which belong to any known class of toxin, is unlikely to result in the production (directly or indirectly) of a novel toxin. This includes the production of such a compound via the site of insertion or the production of a fusion protein. In reference to allergenicity, the introduced glutenin proteins are in a protein class containing allergens which is already present in non-GM wheat. However, the GM wheat will not be used for human food. Conclusion: Risk scenario 1 is not identified as a substantive risk due to the proposed limits and controls designed to minimise exposure of people and other organisms to the GM plant material and the lack of known toxicity of the introduced proteins. Therefore, this risk could not be greater than negligible and does not warrant further detailed assessment. Chapter 2 – Risk assessment 18 DIR 130 – Risk Assessment and Risk Management Plan 2.4.2 Office of the Gene Technology Regulator Risk scenario 2 Risk source Introduced genes for improved grain quality Causal pathway Dispersal of GM seed outside trial limits Growth of GM plants Expression of genes in GM plants Spread and persistence of populations of GM plants outside trial limits Exposure of people or other organisms to GM plant material Potential harm Allergic reactions in people or toxicity in people and other organisms Reduced establishment and yield of desirable plants Reduced biodiversity Risk source The source of potential harm for this postulated risk scenario is the introduced genes for improved grain quality. Causal pathway The grain quality improvement genes are expressed in plant tissues. If seed was dispersed outside the trial site or persisted at the site, this seed could germinate and give rise to plants expressing the introduced genes. These plants could spread and persist in the environment outside the trial limits and people and other organisms may be exposed to GM plant materials. Dispersal of GM plant material outside the limits of the trial site could occur through the activity of people (including the use of agricultural equipment), the activity of animals such as rodents, herbivores and birds, through extremes of weather such as flooding or high winds, or persistence at the site once the trial has finished. Wheat lacks seed dispersal characteristics such as stickiness, burrs and hooks, which can contribute to seed dispersal via animal fur (Howe & Smallwood 1982). The intended introduced trait of improved grain quality is not expected to alter these characteristics of seeds. Seed dispersal for wheat through endozoochory (the ingestion and excretion of viable seeds) has not been reported. Nevertheless, it cannot entirely be discounted that wheat seeds could be dispersed and germinate after passage through the digestive system of some mammals or birds. For example, viable wheat seeds have been detected in cattle dung (Kaiser 1999). Seeds which survive chewing and digestion by animals are typically small and dormant (Malo & Suárez 1995). Corellas were shown to excrete some viable wheat seeds, although the proportion is extremely low (Woodgate et al. 2011). Kangaroos, rabbits and rodents are known pests of wheat crops, and cattle or sheep may graze cereals. The site is fenced, limiting the possibility of seed dispersal by any large animals such as cattle, sheep and kangaroos. Rabbits favour soft, green, lush grass (Myers & Poole 1963) and select the most succulent and nutritious plants first (Croft et al. 2002). Although viable seeds from a variety of plant species have been found in rabbit dung, viable wheat seeds were not among them (Malo & Suárez 1995). Other studies have shown that generally very few viable seeds are obtained from rabbit dung (Welch 1985; Wicklow & Zak 1983). Rodents are opportunistic feeders and their diet include seeds and other plant material (Caughley et al. 1998). They may not only eat and destroy seed at the seed source but also hoard seeds (AGRI-FACTS 2002), which increases the possibility of seed dispersal. Only a very limited amount of rodent activity has been observed at trial sites under other GM field trial licences. Characteristics that influence the spread (dispersal of the plant or its genetic material) and persistence (establishment, survival and reproduction) of a plant species determine the degree of its Chapter 2 – Risk assessment 19 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator invasiveness. These characteristics include the ability to establish in competition with other plants, to tolerate standard weed management practices, to reproduce quickly, prolifically and asexually as well as sexually, and to be dispersed over long distances by natural and/or human means. Baseline information on the weediness of wheat, including factors limiting the spread and persistence of non-GM plants of these species, is given in The Biology of Triticum aestivum L. em Thell (Bread Wheat) (OGTR 2008). In summary, wheat has some characteristics of invasive plants, such as being capable of out-crossing (although it is predominantly self-pollinating) and the ability to germinate or to produce seed in a range of environmental conditions. However, it lacks most of the characteristics that are common to invasive plants, such as the ability to produce a persisting seed bank, rapid growth to flowering, continuous seed production as long as growing conditions permit, high seed output, high seed dispersal and long-distance seed dispersal (Keeler 1989). In addition, wheat has been bred to avoid seed shattering, and white wheat cultivars have little seed dormancy (OGTR 2008). The expected phenotypic difference between the GM wheat lines and their non-GM progenitors is improved grain quality. This introduced trait is not expected to alter the reproductive or dispersal characteristics of the GM plants. In reference to competitive ability, there is the potential for the GM plants to have an increased distribution in the natural environment and agricultural settings. Although the performance of the GM plants in the field is yet to be determined (which will act as an indication of their performance in natural environments), the trait of improved grain quality cannot readily be interpreted as a cue for the GM plants to increase their invasiveness. The techniques of conventional breeding (eg selection of plants amongst available germplasm, wide crosses, mutagenesis) have been used to produce varieties of wheat that possess a range of traits. This experience is a useful backdrop against which to view the potential invasiveness of the GM plants with improved grain quality in this application. Crosses with wild relatives have been used to transfer useful genes to crop plants (Goodman et al. 1987; Hajjar & Hodgkin 2007; Maxted & Kell 2009; Prescott-Allen & Prescott-Allen 1998). Wheat is a crop where such transfer has been most fruitful. Wild relatives of wheat that have been exploited as sources of genes include a whole range of Aegilops species (eg Ae. tauschii, Ae. speltoides, Ae. squarrosa), Triticum species (eg T. turgidum subsp. dicoccoides and T. monococcum) and Thinopyrum bessarabicum. Mutagenesis has also been used to generate a number of varieties of cereals (and many other crops) that have a various traits (Ahloowalia et al. 2004; Cheema et al. 1999; Tomlekova 2010)(FAO/IAEA database of mutation enhanced technologies for agriculture). An increase in the quantity of seed storage proteins in seeds could increase the fitness of the seeds, this in turn increasing the ability of the GM wheat plants to spread and persist. However, no wheat plant that has been generated by any form of conventional breeding has been demonstrated to have increased invasiveness. On the basis of this experience, the genetic modifications are not expected to increase the potential invasiveness of GM plants relative to non-GM plants. De-domestication, the evolutionary loss of traits gained under domestication, is frequently found amongst the small number of known examples of the evolution of invasiveness amongst existing crop plants, this being most obviously reflected in the acquisition of the ability to more readily disperse seed or fruits (Ellstrand et al. 2010). The only known case of invasiveness in wheat is in Tibet, where ‘semi-wild’ wheat (presumably the result of de-domestication) has shattering heads (brittle rachis) and hulled seeds (toughened glumes preventing free threshing) (Ayal & Levy 2005). Both these traits are absent from domesticated wheat, underlining their importance in marking the boundary between cultivated and weedy forms of this plant; shattering allows easy seed dispersal in the wild, while the hull around grains protects the grain from abiotic and biotic stresses. If the GM wheat were to lose the trait of non-shattering heads, it would be expected to spread and persist to a greater degree than non-GM commercial varieties, its seed being lost before harvest and contributing greater amounts of seed to a seed bank. As a result, it would be less dependant on human intervention for dispersal. However, there is no reason to believe that the introduction of the Chapter 2 – Risk assessment 20 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator genes into wheat is likely to lead to a reversion of either the non-shattering or hull-less traits. The obvious rarity of the natural loss of the non-shattering and hull-less traits in conventionally bred commercial wheat varieties indicate their genetic stability. Further, none of the introduced genes are amongst those known to be associated with shattering or the toughness of the glumes, the latter also being a marker of domestication (Sang 2009). The proposed limits and controls of the trial would minimise the likelihood of spread and persistence of the GM plants. The small size (up to 0.06 ha per year) will limit the potential for dispersal of GM plant material and exposure to this material. The proposed trial site is surrounded by a fence and only approved staff with appropriate training will have access, this minimising potential for dispersal of seed and exposure to plant material by grazing livestock and people. Dispersal of GM plant material by authorised people entering the proposed trial site would be further minimised by a standard condition of DIR licences which requires the cleaning of all equipment used at the trial site, including clothing. All GM plant material will be transported in accordance with the Regulator’s transport guidelines, which will minimise the opportunity for its dispersal. Furthermore, extra conditions associated with growing the GM plants in the multiuser NGNE facility would also reduce the likelihood of GM plant material being spread. Monitoring of the trial site for volunteers once the GM wheat has been harvested is expected to minimise the likehihood of persistence. Limits and controls are further discussed in Chapter 3. Potential harm If GM wheat plants were to establish beyond the trial limits, they could potentially cause one or more of the harms outlined in Section 2.3 of this chapter. As discussed in risk scenario 1, the introduced gene products are not expected to be toxic to humans or other organisms. Although glutenins have been associated with allergenicity, there is uncertainty whether or not their expression in wheat (the organism from which they derive) would affect the endogenous allergenicity of that plant. With respect to the environment, spread and persistence of the GM plants could reduce the establishment of desired plants. In turn, this could lead to the fragmentation of the habitats of other plants, decreasing the probability of these plants (and the animals that live amongst these plants) maintaining effective breeding populations. As such, there may be a reduction in the biodiversity in regions where the GM plants grew. In reference to native habitats, it must nevertheless be appreciated that there would have to be large numbers of GM plants before the establishment of native plants was affected. In an agricultural setting, a persistent seed bank of GM wheat could reduce the establishment and yield of subsequent crops. The only expected phenotypic difference between GM wheat and non-GM wheat is altered levels of glutenin. There is no reason to believe that such a trait will have any effect upon the ability of the GM plants to spread and persist, thereby producing any environmental harms. As discussed in Chapter 1, no phenotypic differences were observed between GM wheat and non-GM wheat when grown in glasshouses, and a standard condition of a licence for a field trial would be that the applicant immediately notify the OGTR of any unintended effects. It is important to note that no commercially released variety of wheat that is the product of any form of conventional breeding has been recorded to have negatively impacted the environment (or the health of animals and/or animals), beyond that normally associated with these cereals, and subsequently flagged as an environmental weed. These conventionally generated varieties represent a wide range of traits (Goodman et al. 1987; Hajjar & Hodgkin 2007; Maxted & Kell 2009; Prescott-Allen & Prescott-Allen 1998). Therefore, the introduction into wheat of any of the genes that are the subject of this application is unlikely to produce a plant that poses a risk to the environment that is any different from the many wheat plants produced by conventional breeding (National Research Council 1989). Chapter 2 – Risk assessment 21 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Conclusion: Risk scenario 2 is not identified as a substantive risk due to the proposed limits and controls designed to restrict dispersal of the GM wheat and to minimise exposure of people and other organisms to the GM plant material, and the lack of known toxicity of the introduced proteins. Further, the engineered trait is not associated with weediness. Therefore, this risk could not be greater than negligible and does not warrant further detailed assessment. 2.4.3 Risk scenario 3 Risk source Introduced genes for improved grain quality Causal pathway Dispersal of GM pollen outside trial limits Vertical transfer of introduced genes to other sexually compatible plants, such as commercial varieties of wheat Expression of genes in plants Exposure of people or other organisms to GM plant material Potential harm Allergic reactions in people or toxicity in people and other organisms Reduced establishment and yield of desirable plants Reduced biodiversity Risk source The source of potential harm for this postulated risk scenario is the introduced genes for improved grain quality. Causal pathway The grain quality improvement genes are expressed in the plant tissues. Pollen from the GM plants could be transferred outside of the trial site (eg via wind) and fertilise sexually compatible plants, whether they be non-GM wheat, or plants from another sexually compatible species. Alternatively, if seed was dispersed outside the trial site, plants expressing the introduced genes may grow and subsequently disperse pollen. Hybrid plants possessing the introduced genes may form the basis for the spread of these genes in other varieties of wheat, or other sexually compatible plant species. People and other organisms could be exposed to the proteins expressed from the introduced genes through contact with (including inhalation of pollen) or consumption of GM plant material deriving from the plants to which the genes have been transferred. It should be noted that vertical gene flow per se is not considered an adverse outcome, but may be a link in a chain of events that may lead to an adverse outcome. Baseline information on vertical gene transfer associated with non-GM wheat plants can be found in The Biology of Triticum aestivum L. em Thell (Bread Wheat) (OGTR 2008). Wheat plants are predominantly self-pollinating and the chances of natural hybridisation occurring with commercial crops or other sexually compatible plants are low and decreases with distance to the GM plants. Outcrossing rates decline significantly over distance, with most pollen falling within the first few metres. Rates are also influenced by the genotype of the variety, and environmental conditions, such as wind direction and humidity. Wheat is sexually compatible with many species within the genus Triticum, and in closely related genera such as Aegilops, Secale (rye) and Elytrigia (Chapter 1, Section 6.3). Durum wheat (other than bread wheat, the only Triticum species present in Australia) can cross with wheat, although there are no reports of gene flow beyond 40 m (Matus-Cadiz et al. 2004). Hybrids between wheat and Secale cereale are sterile, but treatment with colchicine doubles the chromosome number and results in a fertile plant, the commercialised Triticale (Knupffer 2009). Natural hybridisation between wheat and Triticale rarely occurs (Ammar et al. 2004; Kavanagh et al. 2010), at least partly due to both species being largely self-fertilising (Acquaah 2007). Elytrigia repens does occur as an introduced plant in Australia, but a review of possible means of pollen-mediated gene flow from Chapter 2 – Risk assessment 22 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator GM wheat to wild relatives in Europe concluded that there was a minimal possibility of gene flow from wheat to Elytrigia spp. (Eastham & Sweet 2002). Species of Aegilops are not known in Australia. Although specific data is lacking, it is likely that hybridisation of wheat with the four native Australasian Triticeae genera never occurs under natural conditions. The proposed limits and controls of the trial would minimise the likelihood of the dispersal of pollen and exposure to GM plant material. For example, the applicant proposes to control related species within 200 m of the trial site. Isolation from related species and other wheat cultivation will greatly restrict the potential for pollen flow and gene transfer. In addition, the applicant proposes to perform post-harvest monitoring and to destroy any volunteer plants found at the site to ensure that no GM wheat remain that could then hybridise with sexually compatible plants. Potential harm If the vertical transfer of the introduced genes from the GM plants caused the resulting plants to spread and persist in the environment to a degree greater than normally found amongst these species, they may produce one or more harms. These are summarised in Section 2.3 of this chapter. People who are exposed to the proteins expressed from the introduced genes or their associated products through contact or consumption of GM plant material may show toxic or allergenic reactions, while organisms may show toxic reactions from consumption of GM plant material. The GM plants may act to reduce the establishment and yield of desired plants and subsequently reduce biodiversity. In the rare event of the vertical transfer of the introduced genetic material from the GM plants to non-GM wheat plants or sexually compatible species, it is expected that this material in the recipient will have the properties that it possesses in the GM wheat parent. As discussed in risk scenario 1, the introduced gene products are not expected to be toxic to humans or other organisms. Although they have been associated with allergenicity, there is uncertainty whether their expression in wheat (the organism from which they derive) would affect the endogenous allergenicity of that plant. There is no reason to expect these gene products to have toxic or allergenic properties in a recipient arising from hybridisation with sexually compatible species that are any different from those found in the GM wheat. The uncertainty with respect to allergenicity would apply to any plants that were the product of hybridisation. Risk scenario 2 summarises the reasons that the introduced genes are unlikely to make the GM wheat lines more weedy, these reasons likewise being applicable to any plants to which the genes are transferred via hybridisation. The traits that have been introduced into the GM plants of this application could become, via vertical gene transfer, combined with traits of other non-GM commercially cultivated wheat plants. However, there is no reason to believe that the resulting plants would possess a level of toxicity or allergenicity greater than that of either parent, or a level of weediness greater than that of either parent. Conclusion: Risk scenario 3 is not identified as a substantive risk due to the proposed limits and controls designed to restrict dispersal of pollen flow from the GM wheat and to minimise exposure of people and other organisms to the GM plant material, and the lack of known toxicity of the introduced proteins. Further, the introduced trait is not associated with weediness, and it is unlikely that any of the characteristics associated with weeds will occur in hybrids with the GM plants. Therefore, this risk could not be greater than negligible and does not warrant further detailed assessment. Chapter 2 – Risk assessment 23 DIR 130 – Risk Assessment and Risk Management Plan 2.4.4 Office of the Gene Technology Regulator Risk scenario 4 Risk source Introduced genes for improved grain quality Causal pathway Dispersal of GM pollen within the NGNE facility Hybridisation of GM plants of this trial with GM plants (including volunteers) of another trial Expression of genes in stacked GM plants Exposure of people or other organisms to GM plant material Potential harm Allergic reactions in people or toxicity in people and other organisms Risk source The source of potential harm for this postulated risk scenario is the introduced genes for improved grain quality. Causal pathway The grain quality improvement genes are expressed in the plant tissues. The NGNE facility is a multiuser facility, where at any one time, more than one licence holder may be conducting trials of different GM plants. Pollen from the GM plants of one trial could inadvertently fertilise other sexually compatible GM plants, including volunteers, inside a site. This pollen could be the result of transfer by an agent (eg wind, insects) from one planting area to another, or derived from volunteer plants from either trial. In late 2014, the only licence that is authorised to trial GM plants in the Katanning NGNE facility that are sexually compatible with those of this application is DIR 128, held by The University of Adelaide. This latter licence authorises the growth of GM wheat and barley with either abiotic stress tolerance or micronutrient uptake. People working in or visiting the NGNE facility could be exposed to the GM hybrid plants or material from the GM hybrid plants, such as pollen. Any animals accessing the site may also be exposed to hybid GM plants. The applicant has requested permission to grow GM wheat from this licence, and any other licence, next to each other provided they are separated by buffer zones of at least 4 m (2m for this trial and 2m for other trial). The RARMP for DIR 094 considered the possibility of hybridisation between wheat plants occurring over these distances, and concluded that it would be minimal. The licence for DIR 128 specifies that if the GM plants of that trial are grown at the Katanning NGNE facility at the same time as sexually compatible GM and non-GM plants from another licence, seed from any plants of DIR 128 must not be used for the development of commercial cultivars. Imposing such a measure on the proposed trial of this application would help minimise the likelihood that the improved grain quality genes would be transferred to other plants and that people and animals would inadvertently be exposed to GM material. The proposed limits and controls would minimise the possibility that any hybrid seed would leave the trial site. These are discussed in risk scenario 2. Therefore, exposure would be restricted to people working at or visiting the trial sites. All GM seed leaving trial sites will be transported in accordance with the Regulator’s transport guidelines, which will minimise the opportunity for its dispersal and exposure of other people and animals. Potential harm Exposure to the proteins expressed from the introduced genes or their associated products through contact or consumption of GM plant material may cause toxicity or allergenicity in humans, or toxicity in other organisms. As discussed in risk scenario 1, the introduced gene products are not expected to be toxic to humans or other organisms. Although they have been associated with allergenicity, there is Chapter 2 – Risk assessment 24 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator uncertainty whether their expression in wheat (the organism from which they derive) would affect the endogenous allergenicity of that plant. The toxicity and allergenicity associated with the introduced genes of other sexually compatible GM plants growing in the NGNE facility would be the subject of the RARMP(s) associated with their licence(s). According to the RARMP for DIR 128, there is no information to suggest that the proteins encoded by the introduced genes of that application are likely to be toxic to people or other organisms, or allergenic to people. Plants that are the product of hybridisation between two GM varieties are unlikely to possess a level of toxicity or allergenicity greater than that of either parent (ie the stacking of genes from different GM plants will be unlikely to generate a plant with a higher level of toxicity or allergenicity than the individual GM parents). Conclusion: Risk scenario 4 is not identified as a substantive risk due to the proposed limits and controls designed to minimise exposure of people and other organisms to the GM plant material and the lack of known toxicity of the introduced proteins. There is no reasonable expectation that the stacking of genes from different GM trials will lead to plant with an increased level of toxicity. Therefore, this risk could not be greater than negligible and does not warrant further detailed assessment. 2.4.5 Risk scenario 5 Risk source Introduced genes for improved grain quality Causal pathway Dispersal of GM pollen within the NGNE facility Hybridisation of GM plants of this trial with GM plants (including volunteers) of another trial Dispersal of plants or viable plant material containing stacked genes outside the trial limits Expression of genes in stacked GM plants Spread and persistence of populations of GM plants outside a trial site Potential harm Reduced establishment and yield of desirable plants Reduced biodiversity Risk source The source of potential harm for this postulated risk scenario is the introduced genes for improved grain quality. Causal pathway The grain quality improvement genes are expressed in the plant tissues. As discussed in risk scenario 4, the NGNE facility is a multiuser facility, where at any one time, more than one licence holder may be conducting trials of different GM plants. Pollen from the GM plants of one trial could inadvertently fertilise other sexually compatible GM plants, including volunteers, inside a site. GM wheat plants with abiotic stress tolerance and/or micronutrient uptake from DIR 128 may be present at the Katanning NGNE facility. If hybridisation occurred between the GM plants of this application and those of DIR 128, the progeny would have the traits of abiotic stress tolerance, micronutrient uptake stacked with improved grain quality. If plant material, which unknowingly was the product of hybridisation between the GM plants of this trial and other GM plants in any site, was purposely taken outside of a trial site, or was inadvertently dispersed outside of a trial site, GM hybrid plants could arise from the germination of seed. These plants then could spread and persist in the environment. However, considering the nature of the traits in DIR 128 and this application, there is no reason to expect that their stacking will significantly increase the invasiveness of the hybrid GM plants beyond those intrinsic to the parent GM plants. Chapter 2 – Risk assessment 25 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Risk scenario 4 reviews the growing of GM plants from different trials adjacent to each other, such as possibly may occur in the NGNE facility, noting the applicant proposes using buffer zones and structuring the licence similar to other licences that have sought to minimise outcrossing between different GM plants growing in close proximity. The proposed limits and controls of the trial would minimise the possibility that seed would leave any trial site or persist at the site once the trial is completed. These are discussed under risk scenario 2. All GM seed would be transported in accordance with the Regulator’s transport guidelines, which would minimise the opportunity for its dispersal. Potential harm If the vertical transfer of genes from the GM plants causes the recipient species to spread and persist in the environment to a degree greater than normally found amongst these species, they may produce one or more harms. These harms were summarised in Section 2.3 of this chapter and risk scenario 2. In particular, the GM plants may act to reduce the establishment and yield of desired plants and biodiversity. As discussed in risk scenario 2, the introduced gene products are unlikely to cause the GM wheat lines to have adverse impacts on the environment greater than those normally associated with non-GM wheat, these reasons being applicable to any plants to which the genes are transferred. The weediness associated with the introduced genes of other sexually compatible GM plants growing in the NGNE facility would be the subject of the RARMP(s) associated with their licence(s). According to the RARMP for DIR 128, there is no information to suggest that the proteins encoded by the introduced genes of that application are likely to increase the weediness in GM wheat. Based upon the above discussed experience of conventional breeding (risk scenario 2), plants that are the product of hybridisation between two varieties are unlikely to have a greater impact on the environment than that of either parent (ie the stacking of genes from different GM plants will be unlikely to generate a plant more harmful than the individual GM parents). Conclusion: Risk scenario 5 is not identified as a substantive risk due to the proposed limits and controls designed to restrict persistence and dispersal of the GM wheat. There is no reasonable expectation that the stacking of genes from different GM trials will lead to plant with a level of weediness greater than that of the progenitor GM plants. Therefore, this risk could not be greater than negligible and does not warrant further detailed assessment. Section 3 Uncertainty Uncertainty is an intrinsic part of risk analysis4. There can be uncertainty about identifying the risk source, the causal linkage to harm, the type and degree of harm, the chance of harm occurring or the level of risk. In relation to risk management, there can be uncertainty about the effectiveness, efficiency and practicality of controls. Risk analysis can be considered as part of a first tier uncertainty analysis, namely a structured, transparent process to analyse and address uncertainty when identifying, characterising and evaluating risk. However, there is always some residual uncertainty that remains. If the residual uncertainty is important and critical to decision making, then this residual uncertainty may be subjected to further analysis (= second tier uncertainty analysis), such as building ‘worst case’ scenarios, or by using meta-analysis where results from several studies are combined. There are several types of uncertainty in risk analysis (Bammer & Smithson 2008; Clark & Brinkley 2001; Hayes 2004). These include: uncertainty about facts: A more detailed discussion of uncertainty is contained in the Regulator’s Risk Analysis Framework available from the Risk Assessment References page on the OGTR website or via Free call 1800 181 030. 4 Chapter 2 – Risk assessment 26 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator – knowledge – data gaps, errors, small sample size, use of surrogate data – variability – inherent fluctuations or differences over time, space or group, associated with diversity and heterogeneity uncertainty about ideas: – description – expression of ideas with symbols, language or models can be subject to vagueness, ambiguity, context dependence, indeterminacy or under-specificity – perception – processing and interpreting risk is shaped by our mental processes and social/cultural circumstances, which vary between individuals and over time. For DIR 130, uncertainty is noted particularly in relation to the characterisation of: Potential increases in toxicity or allergenicity as a result of the genetic modification Potential for increased survivial of the GMOs, including in land uses outside of agriculture, and Potential for an increase in the level of risk as a result of stacking of the introduced genes with those in other sexually compatible GM plants grown in the NGNE facility. Additional data, including information to address the uncertainties identified above, may be required to assess possible future applications for a larger scale trial, reduced containment conditions, or the commercial release of these GM wheat lines if they are selected for further development. Chapter 3, Section 4, discusses information that may be required for future release. Section 4 Risk evaluation Risk is evaluated against the objective of protecting the health and safety of people and the environment to determine the level of concern and, subsequently, the need for controls to mitigate or reduce risk. Risk evaluation may also aid consideration of whether the proposed dealings should be authorised, need further assessment, or require collection of additional information. Factors used to determine which risks need treatment may include: risk criteria level of risk uncertainty associated with risk characterisation interactions between substantive risks. Five risk scenarios were postulated whereby the proposed dealings might give rise to harm to people or the environment. In the context of the control measures proposed by the applicant, and considering both the short and long term, none of these scenarios were identified as substantive risks that could be greater than negligible. The principal reasons for these conclusions are summarised in Table 2 and include: limits on the size, location and duration of the release proposed by Murdoch University controls proposed by Murdoch University to restrict the spread and persistence of the GM wheat plants and their genetic material the genetic modifications are unlikely to give rise to adverse effects on human health and safety or the environment widespread presence of the same and similar genes and proteins in the environment and lack of evidence of harm from them Chapter 2 – Risk assessment 27 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator limited ability and opportunity for the GM wheat plants to transfer the introduced genes to commercial wheat crops or other sexually related species none of the GM plant materials or products will enter human food or animal feed supply chains. Therefore, risks to the health and safety of people, or the environment, from the prosed release of the GM wheat plants into the enviromnet are considered to be negligible. The Risk Analysis Framework (OGTR 2013a), which guides the risk assessment and risk management process, defines negligible risks as insubstantial with no present need to invoke actions for their mitigation. Therefore, no controls are required to treat these negligible risks. Hence, the Regulator considers that the dealings involved in this proposed release do not pose a significant risk to either people or the environment. Chapter 2 – Risk assessment 28 DIR 130 – Risk Assessment and Risk Management Plan Chapter 3 Office of the Gene Technology Regulator Risk management Section 1 Background Risk management is used to protect the health and safety of people and to protect the environment by controlling or mitigating risk. The risk management plan addresses risks evaluated as requiring treatment and considers controls and limits proposed by the applicant, as well as general risk management measures. The risk management plan informs the Regulator’s decisionmaking process and is given effect through licence conditions. Under section 56 of the Act, the Regulator must not issue a licence unless satisfied that any risks posed by the dealings proposed to be authorised by the licence are able to be managed in a way that protects the health and safety of people and the environment. All licences are subject to three conditions prescribed in the Act. Section 63 of the Act requires that each licence holder inform relevant people of their obligations under the licence. The other statutory conditions allow the Regulator to maintain oversight of licensed dealings: section 64 requires the licence holder to provide access to premises to OGTR inspectors and section 65 requires the licence holder to report any information about risks or unintended effects of the dealing to the Regulator on becoming aware of them. Matters related to the ongoing suitability of the licence holder are also required to be reported to the Regulator. The licence is also subject to any conditions imposed by the Regulator. Examples of the matters to which conditions may relate are listed in section 62 of the Act. Licence conditions can be imposed to limit and control the scope of the dealings. In addition, the Regulator has extensive powers to monitor compliance with licence conditions under section 152 of the Act. Section 2 Risk treatment measures for identified risks The risk assessment of risk scenarios listed in Chapter 2 concluded that there are negligible risks to people and the environment from the proposed field trial of GM wheat. These risk scenarios were considered in the context of the scale of the proposed release (Chapter 1, Section 3.1), the proposed containment measures (Chapter 1, Section 3.2), the receiving environment (Chapter 1, Section 6), and considering both the short and the long term. The risk evaluation concluded that no additional controls are required to treat these negligible risks. Section 3 General risk management The limits and controls proposed in the application were important in establishing the context for the risk assessment and in reaching the conclusion that the risks posed to people and the environment are negligible. Therefore, to maintain the risk context, licence conditions have been imposed to limit the release to the proposed size, location and duration, and to restrict the spread and persistence of the GMOs and their genetic material in the environment. The conditions are detailed in the licence and summarised in this Chapter. 3.1 Licence conditions to limit and control the release 3.1.1 Consideration of limits and controls proposed by Murdoch University Sections 3.1 and 3.2 of Chapter 1 provide details of the limits and controls proposed by Murdoch University in their application. These are discussed in the risk scenarios postulated for the proposed release in Chapter 2. Many of these proposed control measures are considered standard for GM crop trials and have been imposed by the Regulator in previous DIR licences. The appropriateness of these controls is considered further below. The duration of the field trial would be confined to three growing seasons. The trial would be limited to a single site with a maximum area of 0.06 ha per year. The small size and duration of the Chapter 3 – Risk management 29 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator trial would limit the potential exposure of humans and other organisms to the GMOs (risk scenario 1). Only authorised personnel would be permitted to deal with the GMOs. A standard licence condition requires all people dealing with the GMOs to be informed of relevant licence conditions. These measures would limit the potential exposure of humans to the GMOs (risk scenario 1). The Katanning NGNE trial site is surrounded by a fence. This will minimise the potential exposure of livestock and other large animals to the GMOs (risk scenario 1) and the potential dispersal of the GMOs by livestock and other large animals (risk scenario 2). A rodent control program is a standard feature of the NGNE facility. Combined with the use of a monitoring zone (below), this measure should both limit exposure of rodents to the GMOs (risk scenario 1) and minimise potential dispersal of GMOs outside the trial site by rodents (risk scenario 2). A licence condition requires that for the period while GMOs are being grown, and until a trial site has been cleaned, measures must be implemented to control rodents within the site. Birds are known to cause damage to cereal crops mostly during germination in autumn, but may feed on the crop at different times including during grain ripening (Temby & Marshall 2003). An extensive search of the literature did not identify any reports of birds other than emus transporting and dispersing wheat seed (eg through the digestive tract or taking panicles containing viable seed) or seedlings from wheat crops. The white wheat varieties have a thin seed coat (Hansen 1994) and are readily digested by birds (Yasar 2003). Therefore, it is considered appropriate that no measures are needed to prohibit the access of birds to the trial site. However, in this respect it should be noted that the NGNE facility at Katanning is covered by bird netting. The applicant proposes to surround the trial site with a monitoring zone of 10 m width within which the growth of plants should be controlled. This zone is a standard requirement of GM wheat licences. Such an area is also an unfavourable habitat for rodents. In summary, the monitoring zone would fulfill the following purposes: to facilitate detection of plants or related species that might hybridise with GM wheat to reduce rodent activity in the NGNE facility. The applicant proposes that the fence around the Katanning NGNE facility would be surrounded by a 190 m isolation zone where no sexually compatible species will be grown. In addition, inspections are to be conducted for related species in the GMO planting area, monitoring zone and isolation zone, and any found must be destroyed prior to flowering. The potential for pollen movement and gene flow between GM wheat and other sexually compatible species has been addressed at some length in DIR 100, DIR 102, DIR 111 and DIR 112. On the basis of the evidence detailed there, including scientific literature on gene flow, international containment measures for GM wheat trials, and the rules for producing basic and certified seed, a width of 200 m is considered adequate to minimise gene flow from the GM wheat plants to other wheat plants or other sexually related species outside the release site. Therefore, the combination of a 10 m monitoring zone, surrounded by a 190 m isolation zone, will manage gene flow to other wheat crops and related species (risk scenario 3). The NGNE facility is designed to accommodate more than one user. Hence, as there is the possibility that separate trials of other sexually compatible GM plants (most obviously wheat) may concurrently take place in the Katanning facility, it is important to consider the issues of vertical gene transfer and mixing of seeds between plants of different trials. The applicant mentions that DIRs 092, 093, 094, which allow the growth of plants from these three different DIRs in close proximity, requires a minimum of 4 m between each (corresponding to a 2 m buffer zone for each DIR). The licence for DIR 112 specifies that other GM plants under a separate licence may be grown within the Merredin NGNE facility provided they are not grown in the location or buffer zone of DIR 112, the latter being 2 m wide. The more recent licence DIR 128 does not exclude the Chapter 3 – Risk management 30 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator growing of other GM wheat plants in the two NGNE facilities. A minimum distance of 4 m (which corresponds to the 2m buffer zones from each trial) should separate the GM plants of this application and any other sexually compatible GM plants that are grown in the Katanning NGNE facility. If any GM plants in this application are grown in the Katanning NGNE facility at a time when sexually compatible GM plants authorised by another licence are concurrently being grown, seed from the GM plants of this application cannot be used in the future development of cultivars for commercial release. These measures would minimise the likelihood of mixing between seed from different trials or vertical gene transfer (risk scenarios 3, 4 and 5), and subsequent spread of, or exposure to, this GM material. The applicant has proposed a number of measures to minimise the persistence of any GM wheat plants and seeds in the seed bank at the release site after harvest of the trial. These measures include post-harvest tillage to the depth of the original planting and irrigation to promote germination of remaining seed. After harvest, area planted to the GM wheat would be monitored at least once every 35 days for a period of 2 years and three irrigations applied. Volunteer plants that emerge would be destroyed before flowering. There is a difference in germination rates between buried grain and grain lying on the surface; grains remaining on the surface, for example following shallow tillage after harvest, can generally easily germinate and become established (Ogg & Parker 2000). Shallow tillage after harvest, combined with irrigation, will germinate much of the seed lying on the surface (Ogg & Parker 2000). However, deep cultivation in certain soil types can reduce seed viability but can also encourage prolonged dormancy in seeds as a result of a cool, moist low oxygen environment (Ogg & Parker 2000; Pickett 1989). It is therefore considered that under Australian conditions, a 2 year time period during which shallow tillage and a number of irrigations are performed, and monitoring conducted on a regular basis with the failure to detect volunteers for a minimum of 6 months prior to the end of the time period, would effectively manage survival and persistence of viable wheat seeds in the soil. It is appropriate the area receive at least 3 irrigations, at intervals of at least 28 days, with the last required irrigation occurring at a time that would promote germination of volunteers within the final volunteer-free period. A period of natural rainfall may be taken as irrigation only with the agreement of the Regulator. Evidence (such as rainfall measurements, photos etc.) that the rainfall has been sufficient to promote germination needs to be provided. Additionally, prior to the last irrigation the area must be tilled. These treatments will ensure seeds are exposed to sufficient moisture and placed at an appropriate depth for germination, as well as encouraging the microbial decomposition of any residual seed. These measures will minimise the persistence of the GMOs in the environment and are included as licence conditions. In considering potential for spread and persistence of the GMOs, it is important to consider the potential dispersal of grain during sowing and harvesting (mechanical dispersal). This is most likely to result in dispersal of grain into the area immediately around the trial. The licence requires that a 2 m buffer zone and any other areas where GM material has been dispersed, including during harvest or threshing, must be monitored to manage the possibility of mechanical dispersal of seed from the trial location and its persistence after the trial. All equipment used in connection with cultivating and harvesting the GMOs are required to be cleaned on site prior to removal. The applicant has stated that any plant material taken off-site for experimental analysis will be transported according to the Regulator’s Guidelines for the transport, storage and disposal of GMOs. These are standard protocols for the handling of GMOs to minimise exposure of people and other organisms to the GMOs (risk scenario 1), dispersal into the environment and gene flow/transfer (risk scenarios 2, 3 4 and 5). This is included as a licence condition. The applicant does not propose using any of the plant material for human or animal consumption. FSANZ conducts mandatory premarket assessments of GM products in human foods. Chapter 3 – Risk management 31 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator As the GM wheat has not been assessed by FSANZ, a condition in the licence prohibits material from the trial from being used for human food or animal feed. 3.1.2 Summary of licence conditions to be implemented to limit and control the release A number of licence conditions have been imposed to limit and control the release, based on the above considerations. These include requirements to: limit the release to the NGNE facility of Katanning, WA, between May 2015 and December 2017 limit the maximum area of planting to 0.06 ha in any year surround the area where GMOs are grown with a 2 m buffer zone implement measures to control rodents within the planting area surround the trial site with a 10 m monitoring zone, maintained in a manner that does not attract or harbour rodents, and in which related species must be prevented from flowering surround the monitoring zone with a 190 m isolation zone, in which no other crops of wheat may be grown, and where growth of related species are controlled harvest the GM wheat plant material separately from other crops clean the areas and equipment after use apply measures to promote germination of any wheat seeds that may be present in the soil after harvest, including irrigation and tillage monitor for at least 24 months after harvest, and destroy any wheat plants that may grow, until no volunteers are detected for a continuous 6 month period destroy all GM plant material not required for further analysis or future trials not use any seed for further planting if other trials of sexually compatible plants are occurring in the NGNE facility concurrently transport and store GM material in accordance with the Regulator’s guidelines not allow GM plant material to be used for human food or animal feed. 3.2 Other risk management considerations All DIR licences issued by the Regulator contain a number of conditions that relate to general risk management. These include conditions relating to: applicant suitability contingency plans identification of the persons or classes of persons covered by the licence reporting structures a requirement that the applicant allows access to the trial site and other places for the purpose of monitoring or auditing. 3.2.1 Applicant suitability In making a decision whether or not to issue a licence, the Regulator must have regard to the suitability of the applicant to hold a licence. Under section 58 of the Act, matters that the Regulator must take into account include: any relevant convictions of the applicant (both individuals and the body corporate) Chapter 3 – Risk management 32 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator any revocation or suspension of a relevant licence or permit held by the applicant under a law of the Commonwealth, a State or a foreign country the capacity of the applicant to meet the conditions of the licence. On the basis of information submitted by the applicant and records held by the OGTR, the Regulator considers Murdoch University suitable to hold a licence. The licence includes a requirement for the licence holder to inform the Regulator of any circumstances that would affect their suitability. In addition, any applicant organisation must have access to a properly constituted Institutional Biosafety Committee and be an accredited organisation under the Act. 3.2.2 Contingency plan Murdoch University is required to submit a contingency plan to the Regulator before planting the GMOs. This plan would detail measures to be undertaken in the event of any unintended presence of the GM wheat outside of the permitted areas. Murdoch University would also be required to provide a method to the Regulator for the reliable detection of the presence of the GMOs or the introduced genetic materials in a recipient organism. This instrument would be required before conducting any of the licenced dealings with the GMOs. 3.2.3 Identification of the persons or classes of persons covered by the licence The persons covered by the licence are the licence holder and employees, agents or contractors of the licence holder and other persons who are, or have been, engaged or otherwise authorised by the licence holder to undertake any activity in connection with the dealings authorised by the licence. Prior to growing the GMOs, Murdoch University is also be required to provide a list of people and organisations who will be covered by the licence, or the function or position where names are not known at the time. 3.2.4 Reporting requirements The licence obliges the licence holder to immediately report any of the following to the Regulator: any additional information regarding risks to the health and safety of people or the environment associated with the trial any contraventions of the licence by persons covered by the licence any unintended effects of the trial. A number of written notices would also be required under the licence that would assist the Regulator in designing and implementing a monitoring program for all licensed dealings. The notices would include: expected and actual dates of planting details of areas planted to the GMOs expected dates of flowering expected and actual dates of harvest and cleaning after harvest details of inspection activities. 3.2.5 Monitoring for Compliance The Act stipulates, as a condition of every licence, that a person who is authorised by the licence to deal with a GMO, and who is required to comply with a condition of the licence, must allow inspectors and other persons authorised by the Regulator to enter premises where a dealing is Chapter 3 – Risk management 33 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator being undertaken for the purpose of monitoring or auditing the dealing. Post-release monitoring continues until the Regulator is satisfied that all the GMOs resulting from the authorised dealings have been removed from the release site. If monitoring activities identify changes in the risks associated with the authorised dealings, the Regulator may also vary licence conditions, or if necessary, suspend or cancel the licence. In cases of non-compliance with licence conditions, the Regulator may instigate an investigation to determine the nature and extent of non-compliance. The Act provides for criminal sanctions of large fines and/or imprisonment for failing to abide by the legislation, conditions of the licence or directions from the Regulator, especially where significant damage to health and safety of people or the environment could result. Section 4 Issues to be addressed for future releases Additional information has been identified that may be required to assess an application for a large scale or commercial release of these GM wheat lines, or to justify a reduction in containment conditions. This includes: additional molecular and biochemical characterisation of the GM wheat lines, particularly with respect to production of potential toxins or allergens additional phenotypic characterisation of the GM wheat lines, particularly with respect to traits that may contribute to weediness. Section 5 Conclusions of the RARMP The risk assessment concludes that this proposed limited and controlled release of GM wheat poses negligible risks to the health and safety of people or the environment as a result of gene technology, and that these negligible risks do not require specific risk treatment measures. However, conditions have been imposed to limit the release to the proposed size, location and duration, and to restrict the spread and persistence of the GMOs and their genetic material in the environment, as these were important considerations in establishing the context for assessing the risks. Chapter 3 – Risk management 34 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator References Acquaah, G. (2007). Principles of Plant Genetics and Breeding. Blackwell Publishing Ltd, Massachusetts AGRI-FACTS (2002). Mice and their control. Report No. Agdex 683, Alberta Agriculture, Food and Rural Development Ahloowalia, B.S., Maluszynski, M., Nichterlein, K. (2004). Global impact of mutation-derived varieties. Euphytica 135: 187-204 Akagawa, M., Handoyo, T., Ishii, T., Kumazawa, S., Morita, N., Suyama, K. (2007). Proteomic analysis of wheat flour allergens. Journal of Agricultural and Food Chemistry 55: 6863-6870 Albani, D., Hammond-Kosack, M.C.U., Smith, C., Conlan, S., Colot, V., Holdsworth, M., Bevan, M.W. (1997). The wheat transcriptional activator SPA: a seed-specific bZIP protein that recognises the GCN4-like motif in the bifactorial endosperm box of prolamin genes. The Plant Cell 9: 171-184 Ammar, K., Mergoum, M., Rajaram, S. (2004). The history and evolution of triticale. In: M Mergoum, H Gomez-Macpherson, eds. Triticale improvement and production. FAO plant production and protection paper No 179, FAO, Rome, Italy pp 1-9. Anderson, O.D., Greene, F.C. (1989). The characterization and comparative analysis of highmolecular-weight glutenin genes from genomes A and B of a hexaploid bread wheat. Theoretical and Applied Genetics 77: 689-700 Anderson, O.D., Greene, F.C., Yip, R.E., Halford, N.G., Shewry, P.R., Malpica-Romero, J.-M. (1989). Nucleotide sequences of the two high-molecular-weight glutenin genes from the Dgenome of a hexaploid bread wheat, Triticum aestivum L. cv Cheyenne. Nucleic Acids Research 17: 461-462 Anjum, F.M., Khan, M.R., Din, A., Saeed, M., Pasha, I., Arshad, M.U. (2007). Wheat gluten: high molecular weight glutenin subunits - structure, genetics and relation to dough elasticity. Journal of Food Science 72: R56-R63 ASCIA (2014). Food Intolerance AVH (2012). The Council of Heads of Australasian Herbaria 2012, Australia's Virtual Herbarium Ayal, S., Levy, A.A. (2005). Wheat domestication and dedomestication - what are the odds? Chapter 11. In: J Gressel, ed. Crop Ferality and Volunteerism pp 167-173. Baar, A., Pahr, S., Constantin, C., Giavi, S., Papadopoulos, N.G., Pelkonen, A.S., Makela, M., Scheiblhofer, S., Thalhamer, J., Weber, M., Ebner, C., Mari, A., Vrtala, S., Valenta, R. (2014). The high molecular weight glutenin subunit Bx7 allergen from wheat contains repetitive IgE epitopes. Allergy 69: 1316-1323 Baar, A., Pahr, S., Constantin, C., Scheiblhofer, S., Thalhamer, J., Giavi, S., Papadopoulos, N.G., Ebner, C., Mari, A., Vrtala, S., Valenta, R. (2012). Molecular and immunological characterization References 35 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator of Tri a 36, a molecular weight glutenin, as a novel major wheat food allergen. The Journal of Immunology 189: 3018-3025 Barkworth, M.E., Jacobs, S.W.L. (2011). The Triticeae (Gramineae) in Australasia. Telopea 13: 37-56 Barragan-Martinez, C., Speck-Hernandez, C.A., Montoya-Ortiz, G., Mantilla, R.D., AanayaJ.-M., Rojas-Villarraga, A. (2012). Organic solvents as risk factor for autoimmune diseases: a systematic review and meta-analysis. PLoS NE 7: e51506, doi:10.1371/journal.pone.0051506 Barrett-Lennard, E.G. (2003). Saltland Pastures in Australia: a Practical Guide., Edition 2nd ed. Land, Water & Wool Sustainable Grazing on Saline Lands Sub-program, Land & Water Australia, Australian Government Battais, F., Richard, C., Jacquenet, S., Denery-Papini, S., Moneret-Vautrin, D.-A. (2008). Wheat grain allergies: an update on wheat allergens. European Annals of Allergy and Clinical Immunology 40: 67-76 Bell, L.W., Wade, L.J., Ewing, M.A. (2010). Perennial wheat: a review of environmental and agrnomic prospects for development in Australia. Crop and Pasture Science 61: 679-690 Blechl, A., Lin, J., Nguyen, S., Chan, R., Anderson, O.D., Dupont, F.M. (2007). Transgenic wheats with elevated levels of Dx5 and/or Dy10 high molecular-weight glutenin subunits yield doughs with increased mixing strength and tolerance. Journal of Cereal Science 45: 172-183 Blechl, A.E., Anderson, O.D. (1996). Expression of a novel high-molecular-weight glutenin subunit gene in transgenic wheat. Nature Biotechnology 14: 875-879 Blechl, A.E., Vensel, W.H. (2013). Variant high-molecular-weight glutenin subunits arising from biolistic transformation of wheat. Journal of Cereal Science 57: 496-503 Bregitzer, P., Blechl, A.E., Fielder, D., Lin, J., Sebesta, P., De Soto, J.F., Chicaiza, O., Dubcovsky, J. (2006). Changes in high molecular weight glutenin subunit composition can be genetically engineered without affecting wheat agronomic performance. Crop Science 46: 15531563 Caughley, J., Bomford, M., Parker, B., Sinclair, R., Griffiths, J., Kelly, D. (1998). Managing Vertebrate Pests: Rodents. Bureau of Rural Sciences (BRS) and Grains Research and Development Corporation (GRDC) Canberra. Cheema, A.A., Awan, M.A., Ali, Y. (1999). Niab-Irri-9; a new salt tolerant and high yielding rice variety. Pakistan Journal of Biological Sciences 2: 869-870 Christensen, A.H., Quail, P.H. (1996). Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Research 5: 213-218 Christensen, A.H., Sharrock, R.A., Quail, P.H. (1992). Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Molecular Biology 18: 675-689 References 36 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Croft, J.D., Fleming, P.J.S., Van de Ven, R. (2002). The impact of rabbits on a grazing system in eastern New South Wales. 1. Ground cover and pastures. Australian Journal of Experimental Agriculture 42: 909-916 de Virgilio, M., Lombardi, A., Caliandro, R., Fabbrini, M.S. (2010). Ribosome-inactivating proteins: from plant defense to tumor attack. Toxin 2: 2699-2737 Delaney, B., Astwood, J.D., Cunny, H., Conn, R.E., Herouet-Guicheney, C., MacIntosh, S., Meyer, L.S., Privalle, L., Gao, Y., Mattsson, J., Levine, M., ILSI International Food Biotechnology Committee Task Force on Protein Safety (2008). Evaluation of protein safety in the context of agricultural biotechnology. Food and Chemical Toxicology 46: S71-S97 Denham, J.M., Hill, I.D. (2013). Celiac disease and autoimmunity: review and controversies. Current Allergy and Asthma Reports 13: 347-353 Dong, G., Ni, Z., Yao, Y., Nie, X., Sun, Q. (2007). Wheat Dof transcription factor WPBF interacts with TaQM and activates transcription of an alpha-gliadin gene during wheat seed development. Plant Molecular Biology 63: 73-84 Dorofeev, V.F. (1969). Spontaneous hybridization in wheat populations of Transcaucasia. Euphytica 18: 406-416 Eastham, K. and Sweet, J. (2002). Genetically modified organisms (GMOs): The significance of gene flow through pollen transfer. Report No. 28, European Environment Agency Copenhagen, Denmark EFSA (2004). Opinion of the scientific panel on genetically modified organisms on the use of antibiotic resistance genes as marker genes in genetically modified plants. The EFSA Journal 48: 1-18 EFSA (2009). Scientific opinion of the GMO and BIOHAZ Panels on the "Use of antibiotic resistance genes as marker genes in genetically modified plants". European Food Safety Authority 1034: 1-82 Ellstrand, N.C., Heredia, S.M., Leak-Garcia, J.A., Heraty, J.M., Burger, J.C., Yao, L., NohzadehMalakshah, S., Ridley, C.E. (2010). Crops gone wild: evolution of weeds and invasives from domesticated ancestors. Evolutionary Applications 3: 494-504 EPA (1996). Plant pesticide inert ingredient CP4 Enolpyruvylshikimate-3-D and the genetic material necessary for its production in all plants. Report No. 61, US Environmental Protection Agency Eriksson, C., Lundberg, M., Tanka, A., Takahashi, H., Morita, E., Ito, K. (2012). High molecular weight glutenin, Tri a 26, is an important allergen component in children with immediate allergy to wheat. Journal of Allergy and Clinical Immunology 129: Supplement, page AB174 FDA (2006). Approaches to establish thresholds for major food allergens and for gluten in food. Threshold Working Group, The Center for Food Safety and Applied Nutrition Fernandez, A., Mills, E.N.C., Lovik, M., Spoek, A., Germini, A., Mikalsen, A., Wal, J.M. (2013). Endogenous allergens and compositional analysis in the allergenicity assessment of genetically modified crops. Food and Chemical Toxicology 62: 1-6 References 37 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Follmer, C., Barcellos, G.B., Zingali, R.B., Machado, O.L., Alves, E.W., Barja-Fidalgo, C., Guimaraes, J.A., Carlini, C.R. (2001). Canatoxin, a toxic protein from jack beans (Canavalia ensiformis), is a variant form of urease (EC 3.5.1.5): biological effects of urease independent of its ureolytic activity. Biochemical Journal 360: 217-224 Forde, J., Malpica, J.-M., Halford, N.G., Shewry, P.R., Anderson, O.D., Greene, F.C., Miflin, B.J. (1985). The nucleotide sequence of a HMW glutenin subunit gene located on chromosome 1A of wheat (Triticum aestivum L.). Nucleic Acids Research 13: 6817-6832 Furtado, A., Henry, R.J., Pellegrineschi, A. (2009). Analysis of promoters in transgenic barley and wheat. Plant Biotechnology Journal 7: 240-253 Gadaleta, A., Blechl, A.E., Nguyen, S., Cardone, M.F., Ventura, M., Quick, J.S., Blanco, A. (2008). Stably expressed D-genome-derived HMW glutenin subunit genes transformed into different durum wheat genotypes change dough mixing properties. Molecular Breeding 22: 267279 Gil-Humanes, J., Piston, F., Gimenez, M.J., Martin, A., Barro, F. (2012). The introgression of RNAi silencing of-gliadins into commercial lines of bread wheat changes the mixing and technological properties of the dough. PLoS ONE 7: e45937.doi:10.1371/journal.pone.0045937 Gil-Humanes, J., Piston, F., Hernando, A., Alvarez, J.B., Shewry, P.R., Barro, F. (2008). Silencing of gamma-gliadins by RNA interference (RNAi) in bread wheat. Journal of Cereal Science 48: 565-568 Goodman, R.M., Hauptli, H., Crossway, A., Knauf, V.C. (1987). Gene transfer in crop improvement. Science 236: 48-54 Hajjar, R., Hodgkin, T. (2007). The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica 156: 1-13 Hammond, B., Kough, J., Herouet-Guicheney, C., Jez, J.M., ILSI International Food Biotechnology Committe Task Force on the Use of Mammalian Toxicology Studies in the Safety Assessment of GM Foods (2013). Toxicological evaluation of proteins introduced into food crops. Critical review in Toxicology 43 (S2): 25-42 Hansen, W.R. (1994). Small grain production for Iowa - Winter. Report No. Pm-1498, Iowa State University, University Extension Haslberger, A.G. (2003). GM food: the risk-assessment of immune hypersensitivity reactions covers more than allergenicity. Journal of Food, Agriculture and Environment 1: 42-45 Hauser, M., Egger, M., Wallner, M., Wopfner, N., Schmidt, G., Ferreira, F. (2008). Molecular properties of plant food allergens: a current classification into protein families. The Open Immunology Journal 1: 1-12 Henkel, J.S., Baldwin, M.R., Barbieri, J.T. (2010). Toxins from bacteria. Experientia Supplementum Volume 100: 1-29 Herman, R.A., Ladics, G.S. (2011). Endogenous allergen upregulation: transgenic vs. tradtionally bred crops. Food and Chemical Toxicology 49: 2667-2669 References 38 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Herouet, C., Esdaile, D.J., Mallyon, B.A., Debruyne, E., Schulz, A., Currier, T., Hendrickx, K., van der Klis, R.-J., Rouan, D. (2005). Safety evaluation of the phosphinothricin acetyltransferase proteins encoded by the pat and bar sequences that confer tolerance to glufosinate-ammonium herbicide in transgenic plants. Regulatory Toxicology and Pharmacology 41: 134-149 Hischenhuber, C., Crevel, R., Jarry, B., Maki, M., Moneret-Vautrin, D.A., Romano, A., Troncone, R., Ward, R. (2006). Review article: safe amounts of gluten for patients with wheat allergy or coeliac disease. Alimentary Pharmacology and Therapeutics 23: 559-575 Howe, H.F., Smallwood, J. (1982). Ecology of Seed Dispersal. Annual Review of Ecology and Systematics 13: 201-228 Kaiser, A.G. (1999). Increasing the utilisation of grain when fed whole to ruminants. Australian Journal of Agricultural Research 50: 737-756 Karalliedde, L. (1995). Animal toxins. British Journal of Anaesthesia 74: 319-327 Kavanagh, V.B., Hall, L.M., Hall, J.C. (2010). Potential hybridization of genetically engineered Triticale with wild and weedy relatives in Canada. Crop Sci 50: 1128-1140 Keeler, K.H. (1989). Can genetically engineered crops become weeds? Bio/Technology 7: 11341139 Keese, P. (2008). Risks from GMOs due to horizontal gene transfer. Environmental Biosafety Research 7: 123-149 Knupffer, H. (2009). Triticeae genetic resources in ex situ Genebank collections. In: C Feuillet, GJ Muehlbauer, eds. Genetics and Genomics of the Triticeae. Springer, Dordrecht pp 31-80. Lamacchia, C., Shewry, P.R., Di Fonzo, N., Forsyth, J.L., Harris, N., Lazzeri, P.A., Napier, J.A., Halford, N.G., Barcelo, P. (2001). Endosperm-specific activity of a storage protein gene promoter in transgenic wheat seed. Journal of Experimental Botany 52: 243-250 Leighty, C.E., Sando, W.J. (1928). Natural and artificial hybrids of a Chinese wheat and rye. Journal of Heredity 19: 23-27 Leon, E., Aouni, R., Piston, F., Rodriguez-Quijano, M., Shewry, P.R., Martin, A., Barro, F. (2010). Stacking HMW-GS transgenes in bread wheat: combining subunit 1Dy10 gives improved mixing properties and dough functionality. Journal of Cereal Science 51: 13-20 Leon, E., Marin, S., Gimenez, M.J., Piston, F., Rodriguez-Quijano, M., Shewry, P.R., Barro, F. (2009). Mixing properties and dough functionality of transgenic lines of a commercial wheat cultivar expressing the 1Ax1, 1Dx5 and 1Dy10 HMW glutenin subunit genes. Journal of Cereal Science 49: 148-156 Li, Y., Wang, Q., Li, X., Xiao, X., Sun, F., Wang, C., Hu, W., Feng, Z., Chang, J., Chen, M., Wang, Y., Li, K., Yang, G., He, G. (2010). Coexpression of the high molecular weight glutenin subunit 1Ax1 and puroindoline improves dough mixing properties in durum wheat (Triticum turgidum L. ssp.durum). PLoS ONE 7: e50057. doi:10.1371/journal.pone.0050057 MacKenzie, S.A., Lamb, I., Schmidt, J., Deege, L., Morrisey, M.J., Harper, M., Layton, R.J., Prochaska, L.M., Sanders, C., Locke, M., Mattsson, J.L., Fuentes, A., Delaney, B. (2007). References 39 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Thirteen week feeding study with transgenic maize grain event DAS-01507-1 in Sprague-Dawley rats. Food and Chemical Toxicology 45: 551-562 Malo, J.E., Suárez, F. (1995). Herbivorous mammals as seed dispersers in a Mediterranean dehesa. Oecologia 104: 246-255 Maruyama, N., Ichise, K., Katsube, T., Kishimoto, T., Kawase, S., Matsumura, Y., Takeuchi, Y., Sawada, T., Utsumi, S. (1998). Identification of major wheat allergens by means of the Escherichia coli expression system. European Journal of Biochemistry 255: 739-745 Matsuo, H., Kohno, K., Niihara, H., Morita, E. (2005). Specific IgE determination to epitope peptides of omega-5 gliadin and high molecular weight glutenin subunit is a useful tool for diagnosis of wheat-dependent exercise-induced anaphylaxis. The Journal of Immunology 175: 8116-8122 Matus-Cadiz, M.A., Hucl, P., Horak, M.J., Blomquist, L.K. (2004). Gene flow in wheat at the field scale. Crop Science 44: 718-727 Maxted, N. and Kell, S.P. (2009). Establishment of a global network for the in situ conservation of crop wild relatives: status and needs. FAO Commission on Genetic Resources for Food and Agriculture, Rome, Italy Meister, G.K. (1921). Natural hybridization of wheat and rye in Russia. Journal of Heredity 12: 467-470 Merriman, T.N. (1996). An acute oral toxicity study in mice with Phosphinothricin Acetyltransferase (PAT) protein. Report No. Study No. DGC-95-A18, Study No. DGC-95-A18, DEKALB Genetics, unpublished. Morais, J.K.S., Gomes, V.M., Oliveira, J.T.A., Santos, I.S., Da Cunha, M., Oliveira, H.D., Oliveira, H.P., Sousa, D.O.B., Vasconcelos, I.M. (2010). Soybean toxin (SBTX), a protein from soybeans that inhibits the life cycle of plant and human pathogenic fungi. Journal of Agriculture and Food Chemistry 58: 10356-10363 Morita, E., Matsuo, H., Chinuki, Y., Takahashi, H., Dahlstrom, J., Tanaka, A. (2009). Fooddependent exercise-induced anaphylaxis-importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis. Allergology International 58: 493-498 Myers, K., Poole, W.E. (1963). A study of the biology of the wild rabbit, Oryctolagus cuniculus (L.), in confined populations IV. Ther effects of rabbit grazing on sown pastures. The Journal of Ecology 52: 435-451 National Research Council (1989). Field testing genetically modified organisms: framework for decisions. Board on Biology, Commission on Life Sciences, National Academies Press, Washington NYNRMP (2011). Tall wheatgrass (Thinopyrum ponticum). Northern and Yorke National Resource Management Plan, Government of South Australia Ogg, A.G. and Parker, R. (2000). Control of volunteer crop plants. Report No. EB 1523, Washington State University Cooperative Extension References 40 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator OGTR (2008). The Biology of Triticum aestivum L. em Thell. (Bread Wheat). Document prepared by the Office of the Gene Technology Regulator, Canberra, Australia OGTR (2013). Risk Analysis Framework. The Office of the Gene Technology Regulator, Canberra, Australia Panda, R., Ariyarathna, H., Amnuaycheewa, P., Tetteh, A., Pramod, S.N., Taylor, S.L., BallmerWeber, B.K., Goodman, R.E. (2013). Challenges in testing genetically modified crops for potential increases in endogenous allergen expression for safety. Allergy 68: 142-151 Pickett, A.A. (1989). A review of seed dormancy in self-sown wheat and barley. Plant Varieties and Seeds 2: 131-146 Pietzak, M. (2012). Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. Journal of Parenteral and Enteral Nutrition 36 Supplement 1: 68S-75S Plessis, A., Ravel, C., Bordes, J., Balfourier, F., Martre, P. (2013). Association study of wheat grain protein composition reveals that gliadin and glutenin composition are trans-regulated by different chromosome regions. Journal of Experimental Botany 64: 3627-3644 Prescott-Allen, R., Prescott-Allen, C. (1998). Genes from the wild: using wild genetic resources for food and raw materials. International Institute for Environment and Development, Earthscan Publications Ltd, London Radauer, C., Breiteneder, H. (2007). Evolutionary biology of plant food allergens. Journal of Allergy and Clinical Immunology 120: 518-525 Ravel, C., Martre, P., Romeuf, I., Dardevet, M., El-Malki, R., Bordes, J., Duchateau, N., Brunel, D., Balfourier, F., Charmet, G. (2009). Nuceotide polymorphism in the wheat transcriptional activator Spa influences its pattern of expression and has pleiotropic effects on grain protein composition, dough viscoelasticity, and grain hardness. Plant Physiology 151: 2133-2144 Romeuf, I., Tessier, D., Dardevet, M., Branlard, G., Charmet, G., Ravel, C. (2010). wDBTF: an integrated database resource for studying wheat transcription factor families. BMC Genomics 11: 185 Salcedo, G., Quirce, S., Diaz-Perales, A. (2011). Wheat allergens associated with baker's asthma. Journal of Investigational Allergology and Clinical Immunology 21: 81-92 Salcedo, G., Sanchez-Monge, R., Diaz-Perales, A., Pacios, L.F. (2008). Review. Plant food allergens: peach non-specific lipid transfer protein Pru p 3 as a model. Spanish Journal of Agricultural Research 6: 30-37 Sang, T. (2009). Genes and mutations underlying domestication transitions in grasses. Plant Physiology 149: 63-70 Sapone, A., Bai, J.C., Ciacci, C., Dolinsek, J., Green, P.H.R., Hadjivassiliou, M., Kaukinen, K., Rostami, K., Sanders, D.S., Schumann, M., Ullrich, R., Vilalta, D., Volta, U., Catassi, C., Fasano, A. (2012). Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Medicine 10: doi:10.1186/1741-7015-10-13 References 41 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Selmi, C., Leung, P.S.C., Sherr, D.H., Diaz, M., Nyland, J.F., Monestier, M., Rose, N.R., Gershwin, M.E. (2012). Mechanisms of environmental influence on human autoimmunity: a national institute of environmental health sciences expert panel workshop. Journal of Autoimmunity 39: 272-284 Shewry, P.R., Halford, N.G. (2002). Cereal seed storage proteins: structures, properties and role in grain utilization. Journal of Experimental Botany 53: 947-958 Shewry, P.R., Halford, N.G., Tatham, A.S. (1992). High molecular weight subunits of wheat glutenin. Journal of Cereal Science 15: 105-120 Shewry, P.R., Halford, N.G., Tatham, A.S., Popineau, Y., Lafiandra, D., Belton, P.S. (2003). The high molecular weight subunits of wheat glutenin and their role in determining wheat processing properties. Advances in Food and Nutrition Research 45: 219-302 Shewry, P.R., Tatham, A.S. (1997). Biotechnology of wheat quality. Journal of the Science of Food and Agriculture 73: 397-406 Steiner, H.Y., Halpin, C., Jez, J.M., Kough, J., Parrott, W., Underhill, L., Weber, N., Hannah, L.C. (2013). Evaluating the potential for adverse interactions within genetically engineered breeding stacks. Plant Physiology 161: 1587-1594 Stirpe, F. (2005). Ribosome-inactivating proteins. Chapter 2. In: RG Wiley, DA Lappi, eds. Molecular Neurosurgery with Targeted Toxins. Humana Press pp 9-29. Sugiyama, T., Rafalski, A., Peterson, D., Soll, D. (1985). A wheat HMW glutenin subunit gene reveals a highly repeated structure. Nucleic Acids Research 13: 8729-8737 Sutcliffe, J.G. (1979). Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harbor Symposia on Quantitative Biology 43 Pt 1: 77-90 Tatham, A.S., Shewry, P.R. (2008). Allergens to wheat and related cereals. Clinical and Experimental Allergy 38: 1712-1726 Temby, I. and Marshall, D. (2003). Reducing cockatoo damage to crops. Report No. Landcare Notes LC0009, State of Victoria, Department of Sustainability and Environment Thompson, R.D., Bartels, D., Harberd, N.P. (1985). Nucleotide sequence of a gene from chromosome 1D of wheat encoding a HMW-glutenin subunit. Nucleic Acids Research 13: 68336846 Tomlekova, N.B. (2010). Induced mutagenesis for crop improvement in Bulgaria. Plant Mutation Reports 2: 4-27 Vasconcelos, I.M., Morais, J.K.S., Siebra, E.A., Carlini, C.R., Sousa, D.O.B., Beltramini, L.M., Melo, V.M.M., Oliveria, J.T.A. (2008). SBTX, a new toxic protein distinct from soyatoxin and other toxic soybean [Glycine max] proteins, and its inhibitory effect on Cercospora sojina growth. Toxicon 51: 952-963 Vasconcelos, I.M., Trentim, A., Guimaraes, J.A., Carlini, C.R. (1994). Purification and physicochemical characterization of soyatoxin, a novel toxic protein isolated from soybeans (Glycine max). Archives of Biochemistry and Biophysics 312: 357-366 References 42 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Wang, H.Y., Liu, D.C., Yan, Z.H., Wei, Y.M., Zheng, Y.L. (2005). Cytological characteristics of F2 hybrids between Triticum aestivum L. and T. durum Desf. with reference to wheat breeding. Journal Applied Genetics 46: 365-369 Weber, N., Halpin, C., Hannah, L.C., Jez, J.M., Kough, J., Parrott, W. (2012). Editor's choice: crop genome plasticity and its relevance to food and feed safety of genetically engineered breeding stacks. Plant Physiology 160: 1842-1853 Weibo, J., Jin, L., Fangli, W., Aiguang, G. (2009). Cloning of high molecular weight gluten subunit promoter and study on its function in wheat. Brazilian Archives of Biology and Technology 52: 265-270 Welch, D. (1985). Studies in the grazing of heather moorland in north-east Scotland. IV. Seed dispersal and plant establishment in dung. The Journal of Applied Ecology 22: 461-472 Wicklow, D.T., Zak, J.C. (1983). Viable grass seeds in herbivore dung from a semi-arid grassland. Grass and Forage Science 38: 25-26 Wieser, H. (2007). Chemistry of gluten proteins. Food Microbiology 24: 115-119 Wieser, H., Zimmermann, G. (2000). Importance of amounts and proportions of high molecular weight subunits of glutenin for wheat quality. European Food Research and Technology 210: 324330 Wink, M. (2009). Mode of action and toxicology of plant toxins and poisonous plants. Mitteilungen aus dem Julius Kuhn-Institut 421: 93-112 Wink, M., Van Wyk, B.-E. (2008). Mind-altering and poisonous plants of the world: a scientifically accurate guide to 1200 toxic and intoxicating plants. Timber Press, Portland and London Woodgate, J.L., Steadman, K.J., and Buchanan, K.L. (2011). A study of seed viability following consumption by birds. Unpublished final report submitted to the OGTR. Wu, W., Sun, R. (2011). Toxicological studies on plant proteins: a review. Journal of Applied Toxicology 32: 377-386 Yasar, S. (2003). Performance of broiler chickens on commercial diets mixed with whole or ground wheat of different varieties. International Journal of Poultry Science 2: 62-70 Yokooji, T., Kurihara, S., Murakami, T., Chinuki, Y., Takahashi, H., Morita, E., Harada, S., Ishii, K., Hiragun, M., Hide, M., Matsuo, H. (2013). Characterization of causative allergens for wheatdependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat proteins in facial soap. Allergology International 62: 435-445 Zhang, Y., Tang, J., Zhang, Y., Yan, J., Xiao, Y., Zhang, Y.X.X., He, Z. (2011). QTL mapping for quantities of protein fractions in bread wheat (Triticum aestivum L.). Theoretical and Applied Genetics 122: 971-987 Zheng, W., Peng, Y., Ma, J., Appels, R., Sun, D., Ma, W. (2011). High frequency of abnormal high molecular weight glutenin alleles in Chinese wheat landraces of the Yangtze-river region. Journal of Cereal Science 54: 401-408 References 43 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Appendix A Summary of submissions from prescribed experts, agencies and authorities5 Advice received by the Regulator from prescribed experts, agencies and authorities on the consultation RARMP is summarised below. All issues raised in submissions that related to risks to the health and safety of people and the environment were considered in the context of the currently available scientific evidence and were used in finalising the RARMP that formed the basis of the Regulator’s decision to issue the licence. Summary of issues raised Comment Agrees with the overall conclusions of the RARMP. Noted. Should consider further data on allergenicity that may be required for any future commercial release application. Future data requirements identified in Section 4, Chapter 3 lists potential allergens produced by the GM wheat lines. Agrees with the Regulator’s assessment that this trial poses negligible risks to human health and safety or the environment under the proposed conditions of the RARMP. Noted. Satisfied with the conclusions of the RARMP and has no technical comments. Noted. No objection to the issue of a licence for DIR 130. Noted. Notes that the licence will prohibit the use of material from the trials for human or animal consumption. No further comments on the licence application at this stage. Noted 5 Prescribed agencies include GTTAC, State and Territory Governments, relevant local governments, Australian Government agencies and the Minister for the Environment. Appendix A 44 DIR 130 – Risk Assessment and Risk Management Plan Office of the Gene Technology Regulator Appendix B Summary of submissions from the public The Regulator received five submissions from the public on the consultation RARMP. The issues raised in these submissions are summarised in the table below. All issues raised in the submissions that related to risks to the health and safety of people and the environment were considered in the context of currently available scientific evidence in finalising the RARMP that formed the basis of the Regulator’s decision to issue the licence. Abbreviations: Issues raised: AT: Alternative technology; C: Containment; CC: Consumer choice; CP: Consultation process; D: Detection of GMOs; E: Environment; Ec: Economic issues; F: Food safety; FL: Food labelling; H: Human health; L: liability; LC: Licence condition; M: Monitoring; MT: Marketing and trade; R: Research; RA: Risk analysis; S: Segregation Other abbreviations: Act: the Gene Technology Act 2000; APVMA: Australian Pesticides and Veterinary Medicines Authority; Ch: Chapter; FSANZ: Food Standards Australia New Zealand; GM: Genetically modified; GMO: Genetically modified organism; Regulator: the Gene Technology Regulator. Submission Issue number 1 Appendix B Summary of issues raised Comment C Opposed to all trials of DIR 130 GM wheat unless they are in a totally confined area from which no pollen can escape. Wheat plants are predominantly self-pollinating and the chances of natural hybridisation occurring with commercial crops or other sexually compatible plants are low. Licence conditions require isolation from other wheat plants to minimise the opportunity of pollen flow. H Claims that numerous studies (eg Seralini, Carman) have demonstrated with animals the serious possible health effects from eating GM food. More and more doctors in the US are advising patients to avoid all GM foods. Consumers, once they have recognised the effect GM food can have on their gut and consequently their health, will never accept GM food. No GM foods have been proven safe despite the claims of the industry. No products from this GM wheat trial will enter the human food supply. The RARMP concluded that the limited and controlled release of the GM wheat lines included in this application poses negligible risks to the health and safety of people. FSANZ has critically evaluated the studies cited as evidence of adverse effects from GM foods and concluded that these studies provided no grounds to revise its conclusions on the safety of food derived from the previously approved GM crops (details available on the FSANZ website). C, Ec, MT, FL Claims that GM food will always only be used for industrial purposes or animal feed with lower prices due to strong consumer resistance. Concerned that the whole wheat harvest in Australia would have to be labelled as GM due to contamination if GM wheat is released. WA wheat is already at risk due to testing of GM wheat in the open environment. Also claims that the only reason for the continued imposition of GM food on the general public is either corporations or governments which believe they will make a lot of money. Strict licence conditions have been imposed to minimise the likelihood of the GM wheat mixing with non-GM wheat. Issues of marketing and trade implications are outside the scope of the Regulator’s assessment required by the Act. These issues are the responsibility of the State Governments and the industry. FSANZ is responsible for labelling of GM foods. 45 DIR 130 – Risk Assessment and Risk Management Plan Submission Issue number Office of the Gene Technology Regulator Summary of issues raised Comment E GM canola is spreading down the roads in areas of the wheat belt in WA and this is also a serious problem in the US. GM wheat will make the weed problem worse. The DIR 130 licence imposes strict controls that restrict the spread and persistence of the GMOs in the environment, including monitoring of the trial site to ensure no GMOs remain following completion of the trial. AT Claims that selective breeding and marker assisted breeding have been proven to be far more successful than any GM breeding and commodities from such technologies would be welcomed by the public. The comparison and choice between technologies are outside the scope of assessment by the Regulator. 2 CP Requests that the trial not proceed and that all GM trials be specifically approved by the citizens and residents of the council governing the area in which the trial GM wheat will be planted. As required by the Act, the Regulator consulted with the Local Government Area where the GM wheat is proposed to be planted Consultation with the public was also undertaken, which involved advertising in the Australian and the WA Farm Weekly newspapers, the Australian Government gazette and on the OGTR website, as well as emailing people on OGTR’s client register. The Regulator considered the issues raised in the submissions related to human health and safety and the environment before making the decision to issue the licence. 3 H, E, R, M, PU Urges the OGTR to reject the DIR 130 application. Advocates a GM freeze - no new GM foods approved; no new GM crop approvals and no new GM crop growing areas. During the GM freeze, the safety of GM crops on sale must be re-assessed and data on yield, pesticide use and effects on biodiversity must be investigated and the results of these studies made public. Before any more open field trials are approved, thorough testing, monitoring, surveillance, problem reporting and recall mechanisms are needed. The Regulator has prepared a comprehensive RARMP, in accordance with the requirements of the Act, and includes a comprehensive and critical assessment of data supplied by the applicant, together with a thorough review of other relevant national and international scientific literature. The RARMP concluded that risks to human health and safety and the environment, including effects on biodiversity are negligible as a result of this limited and controlled release of GM wheat. No products from this GM wheat trial will enter the human food supply. The DIR 130 licence imposes conditions for monitoring the trial site for 24 months after field trials have completed to ensure no GMOs remain before the site can be signed off. Prior to conducting any dealings, the licence holder is also required to prepare a contingency plan for measures to be taken in the event of the unintended presence of the GMOs outside an area that must be inspected, including reporting, recovering, destroying and inspecting any of the GMOs. The licence requires that any risks to health and safety of people or the environment or unintended effects be reported. The Regulator is able to suspend, cancel or vary a licence if risks associated with the field trial are identified. The regulation of agricultural chemicals in Australia is the responsibility of APVMA. Appendix B 46 DIR 130 – Risk Assessment and Risk Management Plan Submission Issue number Appendix B Office of the Gene Technology Regulator Summary of issues raised Comment F Claims that there is a growing body of evidence which questions the safety of GM food and the American Academy of Environmental Medicine recommends avoiding GM foods when possible. No products from this GM wheat trial will enter the human food supply. FSANZ is responsible for human food safety assessment, including GM food. C, L It remains unresolved who is responsible for the control and clean-up of the spread and spill of GM seed and crops. There is little or no education and training for non-GM farmers, government instrumentalities and the general public. Claims that escapes from GM trials have occurred and attempts to eradicate the resulting GM weeds post-trial is likely to continue for many years, potentially forever. For limited and controlled release, the licence holder is responsible for the control and clean-up of the spread or spill of GM material outside the trial sites. Prior to conducting any dealings, the licence holder is required to prepare a contingency plan for measures to be taken in the event of the unintended presence of the GMOs outside an area that must be inspected, including reporting, recovering, destroying and inspecting any of the GMOs. Monitoring and management of GM wheat volunteers beyond the trial period are discussed in Chapter 3 of the RARMP. The licence requires at least two years of monitoring the trial site and destroying volunteers before the site can be considered for sign-off. This would effectively manage survival and persistence of viable wheat seeds in the soil. In the case of commercial release of GM crops, matters relating to segregation and coexistence of different farming systems are the responsibility of the States, Territories and industry. C OGTR "Buffer zones" of 200 metres around the crops are inadequate to stop the spread of transgenic material. Contamination has also occurred during transport of GM material. Based on scientific literature, a 200 m isolation zone is considered in the RARMP as adequate to limit gene flow to non-GM wheat. This has been shown to be an effective containment measure for GM wheat trials under previously issued licences. The licence holder is required to comply with DIR 130 licence conditions for safe transport of GM material. C Claims that GM crops are proven to contaminate with examples from: 1) In 2013, unapproved GM wheat from trials years earlier was found growing in an Oregon field in the USA, which resulted in rejections of US wheat shipments to a number of Asian destinations; 2) China intercepted & destroyed shipments of GM corn seed that attempted to bypass Chinese environmental & food safety tests; 3) most of the GM canola trial sites in Tasmania have still not been cleaned after 10 years. As this application is for a limited and controlled release (field trial), strict licence conditions are imposed to restrict the spread and persistence of the GMOs and their genetic material in the environment. These include conditions to isolate the trial site from other wheat and crops, cleaning of equipment used with GM plant materials, secure transport and storage of GM plant materials, and post-harvest monitoring at the trial site to ensure all GM plants are destroyed. Similar conditions have been effective for other GM wheat trials conducted in Australia. C Concern has been raised about the maintenance of GM wheat research at Merredin, WA, following an ABC report in June 2014. The OGTR is aware of the claims made in the media and has investigated them. The Regulator was satisfied that the issue did not represent a risk to people and the environment and work with GMOs undertaken in the Merredin facility is managed according to licence conditions. The licence holder intends to plant at Katanning facility and not at Merredin. 47 DIR 130 – Risk Assessment and Risk Management Plan Submission Issue number 4 Appendix B Office of the Gene Technology Regulator Summary of issues raised Comment MT GM wheat is not approved or grown commercially anywhere in the world. Claims that there is no apparent market for GM wheat products and the WA Farmers Association Grains Group has commented that GM wheat would do more damage to WA wheat markets than good. A petition to stop Australian wheat trials also happened in New Caledonia. See comments for Submission 1 regarding issues relating to marketing and trade. S, MT The Marsh v Baxter appeal is set to be heard in the Court of Appeal of the WA Supreme Court March 23-25, 2015. Assessment of this application must be delayed until after the appeal judgement as it could contribute significantly to the risk assessment. The Marsh vs Baxter legal case relates to commercially approved GM crops and involves segregation and marketing issues, not health and safety issues, and as such is outside the scope of the Regulator’s assessment required by the Act. C Based on a recent ABC report, regulatory See comments for Submission 3 regarding this oversight, surveillance and monitoring at the issue. NGNE is poor, creating a significant risk of escape of GM organisms. D The community must be educated on the trials before release so that the community can be vigilant about escapes. Gene test kits must be available so that GM contamination outside the trial area can be tested. This is a limited and controlled field trial with strict licence conditions to minimise the likelihood of escape from the trial limits. Field trial sites where GMOs are planted are published on the OGTR website. Prior to conducting any dealings with the GMOs, the licence holder must provide to the Regulator written methodology to reliably detect the GMOs. M History proves there is a high risk of escapes such as the unapproved GM wheat found in a wheat field in Oregon causing importing countries to place immediate embargoes on GM wheat. This occurred many years after the trial was completed. The DIR130 RARMP must reflect the ongoing surveillance, testing and monitoring beyond the trial period. See comments for Submission 3 regarding containment of GM wheat trials in Australia. Monitoring and management of GM wheat volunteers beyond the trial period are discussed in Chapter 3 of the RARMP and the licence imposes strict measures to minimise the spread and persistence of the GM wheat. 48 DIR 130 – Risk Assessment and Risk Management Plan Submission Issue number 5 Summary of issues raised Comment H Long-term animal feeding studies with human health end-points must be conducted in a confined controlled setting before intentional release of the GM organisms to the environment. This has not been done and a theoretical argument of “substantial equivalence” cannot safely be assumed until such testing has been done to determine unintended consequences and to quantify risks of side-effects. Harm is indicated from GM organisms and associated pesticides and herbicides, for example: the American Academy of Environmental Medicine recommends avoiding GM foods when possible A peer-reviewed paper reported harm to pigs fed GM feed Glyphosate has been found in mother’s breast milk, urine and babies’ feeding tubes. FSANZ is responsible for human food safety assessment, including GM food. An analysis of the pig feeding study has been published by FSANZ (http://www.foodstandards.gov.au/consumer/gmfoo d/Pages/Response-to-Dr-Carman%27s-study.aspx). Issues relating to herbicide use are outside the scope of the Regulator’s assessments. The APVMA has regulatory responsibility for agricultural chemicals, including herbicides, in Australia. CC The world has already rejected GM wheat from earlier attempts in the US and Canada in 2004 to commercialise it. Consumers do not want to place their families at risk. Application DIR 130 must be rejected. Consumer choice is outside the scope of responsibility of the Regulator. This is a GM wheat field trial and not a commercial release. No products from this field trial will enter the human food supply. MT Strongly opposed to this high risk trial and calls for a GM freeze. It jeopardizes our major export market, and undermines Australia's standing and credibility overseas and at home. Issues of marketing and trade implications are outside the scope of the Regulator’s assessment required by the Act. These issues are the responsibility of the State Governments and the industry. R, H, E Claims that the OGTR and FSANZ are inextricably linked and share a joint accountability. Neither can be relied on because the government has forced them to be reliant on GM company researchers for safety testing. Thorough, peer reviewed, long term epidemiological studies by independent medical researchers remain to be done on human health and safety, and environmental safety. When preparing a RARMP, the Regulator considers not only information provided by the applicant, but also published scientific literature, and advice received from a range of Australian government authorities, agencies, experts and the public. Only when satisfied that any risks to human health and the environment can be managed, the Regulator will consider issuing a licence for intentional release of the assessed GMOs into Australian environment. F A dedicated national surveillance system for potential health effects of GM foods needs to be put in place immediately. FSANZ is responsible for human food safety assessment, including GM food. All risks associated with GM crops, eg increased use of herbicides and pesticides, must be incorporated into the risk analysis framework Risks to the health and safety of people and the environment as a result of the release of the GM wheat are considered by the Regulator. Issues related to the use of agricultural chemicals are the responsibility of APVMA. RA Appendix B Office of the Gene Technology Regulator 49