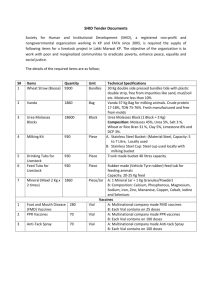
S. No. Item Required Specification Quantity 1. ND+ IB Live Vaccines
advertisement
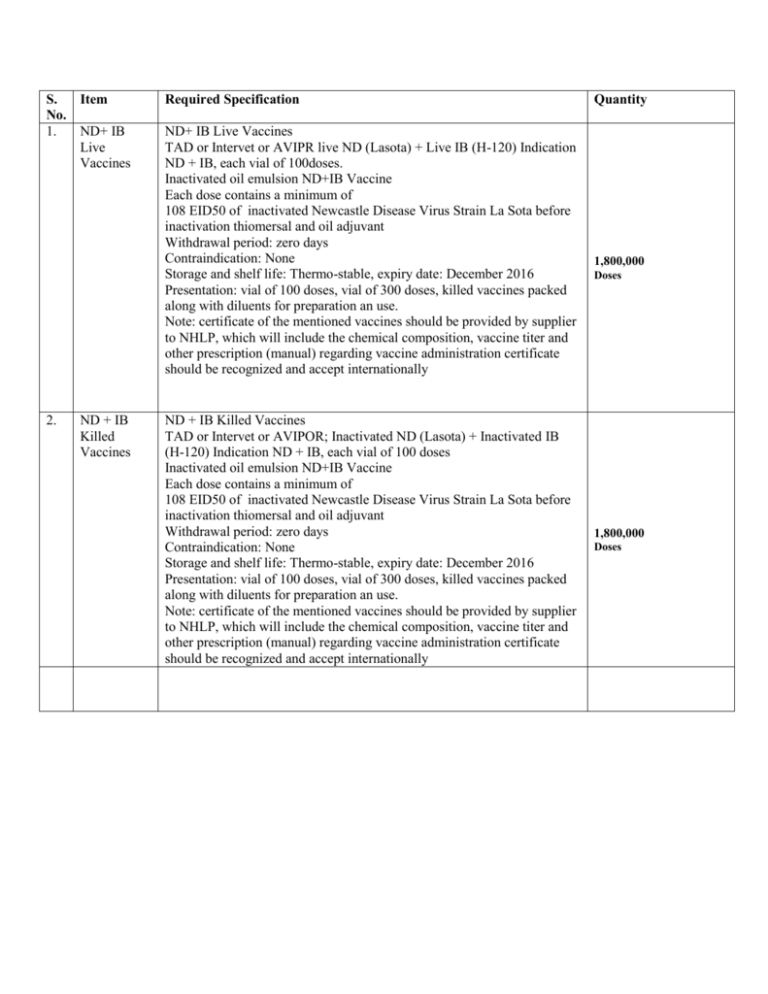
S. Item No. 1. ND+ IB Live Vaccines 2. ND + IB Killed Vaccines Required Specification ND+ IB Live Vaccines TAD or Intervet or AVIPR live ND (Lasota) + Live IB (H-120) Indication ND + IB, each vial of 100doses. Inactivated oil emulsion ND+IB Vaccine Each dose contains a minimum of 108 EID50 of inactivated Newcastle Disease Virus Strain La Sota before inactivation thiomersal and oil adjuvant Withdrawal period: zero days Contraindication: None Storage and shelf life: Thermo-stable, expiry date: December 2016 Presentation: vial of 100 doses, vial of 300 doses, killed vaccines packed along with diluents for preparation an use. Note: certificate of the mentioned vaccines should be provided by supplier to NHLP, which will include the chemical composition, vaccine titer and other prescription (manual) regarding vaccine administration certificate should be recognized and accept internationally ND + IB Killed Vaccines TAD or Intervet or AVIPOR; Inactivated ND (Lasota) + Inactivated IB (H-120) Indication ND + IB, each vial of 100 doses Inactivated oil emulsion ND+IB Vaccine Each dose contains a minimum of 108 EID50 of inactivated Newcastle Disease Virus Strain La Sota before inactivation thiomersal and oil adjuvant Withdrawal period: zero days Contraindication: None Storage and shelf life: Thermo-stable, expiry date: December 2016 Presentation: vial of 100 doses, vial of 300 doses, killed vaccines packed along with diluents for preparation an use. Note: certificate of the mentioned vaccines should be provided by supplier to NHLP, which will include the chemical composition, vaccine titer and other prescription (manual) regarding vaccine administration certificate should be recognized and accept internationally Quantity 1,800,000 Doses 1,800,000 Doses