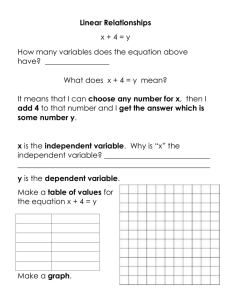
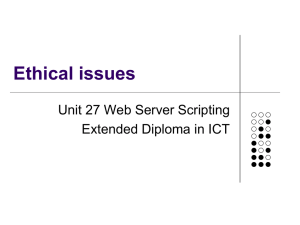
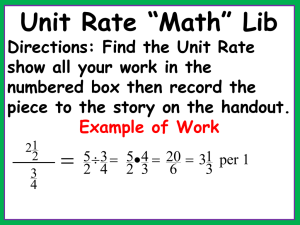
Lyssa Aruda Writ 340 Dr. Ramsey 1 May 2013 Recipe for Success
advertisement

Lyssa Aruda Writ 340 Dr. Ramsey 1 May 2013 Recipe for Success: The Science Behind Your Favorite Cookies Abstract This article details the chemistry behind baking cookies. Baking is a complex chemical process in which each ingredient acts as a reactant and the cookies are the final product. The chemical composition of each reactant, as well as using precise measurements, allows cookies to form. The recipe is a procedure, and each step is important in creating the perfect cookies. The science behind each ingredient and step is explained. Keywords: Chemistry, cookies, baking, procedure Multimedia Suggestions: time lapse of cookies baking. Ex. http://www.youtube.com/watch?v=DrCiXlItdto&feature=endscreen&NR=1 Biography: Lyssa Aruda is currently a sophomore studying chemical engineering with an emphasis in nanotechnology and a minor in art history at the University of Southern California. She is very involved on campus, acting as a Viterbi Student Ambassador, Freshman Academy Coach, and as part of a social sorority. Her favorite memory growing up was baking Christmas cookies with her mom, and this sparked her love of science and chemistry. Introduction No one can resist the allure of fresh baked cookies right out of the oven, the warm, comforting smell of sugar and chocolate wafting through the air, and the chewy moistness of the first bite. First created by Ruth Wakefield by accident in her Bed and Breakfast Toll House Inn in Massachusetts, chocolate cookies have become a staple of American comfort food. [1] However, what most people don’t consider is the science behind their tasty treats. Baking involves numerous complex chemical reactions and physical changes, which cause the assortment of ingredients (reactants) to meld together and create delicious cookies (products). Changing the recipe, or procedure, even a little can have disastrous effects on the final product. This article will explore how the different reactants change during the course of the baking process, and how each affects the cookies. The Recipe Chocolate chip cookie recipes can be reinterpreted as a list of chemical materials followed by a procedure. In chemistry, the mole is the common counting unit. One mole contains 6.022 x 1023 molecules. Thus, the major chemical components of each ingredient can be converted into moles, which is more useful to a chemist. Toll House Chocolate Chip Cookies [1] 2 1/4 cups all-purpose flour 1 teaspoon baking soda 1 teaspoon salt 1 cup (2 sticks) butter, softened 3/4 cup granulated sugar 3/4 cup packed brown sugar 1 teaspoon vanilla extract 2 large eggs 2 cups (12-oz. pkg.) NESTLÉ® TOLL HOUSE® Semi-Sweet Chocolate Morsels Chemical Materials 2.394 mol C4H8O4 0.0355 mol NaHCO3 0.072 mol NaCl 1.084 mol C9H14O6 0.044 mol C12H24O11 0.044 mol C12H24O11 0.031 mol C8H8O3 0.624 mol C6H12O3N2 2.82 mol C4H8O4 Fig 1. The Toll House Original Chocolate Chip Cookie Recipe and the corresponding chemical conversions using stoichiometry. Sources 1, 2 The Ingredients Flour The bulk of most cookies comes from flour. Flour is an integral ingredient for its protein content. The proteins glutenin and gliadin [3] found in flour form long gluten bonds when they come in contact with water. These long chains form a network that gives the cookies structure. During the baking process, cookies puff up from CO2 productions (see leavening agents) and lift the gluten bonds, which then set to create the tender texture of the cookie. Flour with a moderate amount of protein is ideal for cookies because it gives a sturdy structure without creating too extensive of a network that would make the cookie too bread like. Fats Fats like butter, shortening, or oil serve three main purposes: limiting gluten formation, controlling spread, and trapping air bubbles to create rise. In order to regulate the gluten formation in the flour, the fats coat the glutenin and gliadin strands, and they prevent them from attaching to form gluten, which keeps cookies chewier. Using fats that have different melting points helps to control the spread of cookies. Butter has a relatively low, sharp melting point that allows cookies to spread and become thinner. Shortening has a higher melting point because it is one hundred percent fat and has no additives. Butter is only eighty percent fat, with the other twenty percent being water and nonfat milk solids. [4] Mixtures always have a lower melting point, which explains why the use of shortening causes a fluffier cookie. Finally, cookie recipes usually call for room temperature fats to be creamed with sugar. This combination allows sugar crystals to cut into the fat and creates tiny holes, which will aerate the cookie and later trap the CO2 produced by the leavening agent. Without these air bubbles, the cookies spread much more and are thinner and crisper. Sugar Sugar is definitely one of the major ingredients of cookies, however it is not only used for its sweetening properties. Sugar serves two main purposes outside of sweetening. First, long sucrose chains break down into glucose and fructose (Fig. 2), which then forms into long polymer chains. The carbon in these chains reacts in heat, with the surface carbon chains reacting first, to caramelize and create the brown, crunchy outer shell of the cookie. Second, sugars are extremely hygroscopic and trap water. This means that sugar will retain moisture during the heat of the baking process and keep the cookies chewy. Different types of sugar have differing hygroscopic properties. Molasses found in brown sugars is also extremely hygroscopic, making brown sugar a better choice when a chewier texture is desired. Pure white sugar is much more refined and therefore has fewer additives to help it retain water. Eggs Fig 2: Chemical formulas for sucrose and the resulting fructose and glucose. Source: http://cdavies.wordpress.com /2009/01/27/simple-sugars-fructose-glucose-and-sucrose/ Eggs have two components, the yolk, primarily a fat, and the white, primarily a protein. Eggs are beaten into the creamed sugar and butter. The yolk works with the butter to coat the gluten strands and adds the richness and melt associated with cookies. It keeps gluten strands from over forming and allows the cookie to maintain moisture and not become bread like. The protein in the whites, on the other hand, adds to the fluffiness and structure of the cookie. The whites are beaten and whipped into a puff which aerates the cookies and the proteins form a network to help the gluten hold up the cookie. Leavening The most common leavening agents in cookies are either baking soda or baking powder. Essentially, each of these ingredients performs the same task; reacting to create CO2 bubbles to hold up the gluten network and give cookies their puff. Baking soda, or sodium bicarbonate, reacts by the following reaction in heat: 2NaHCO3 H2O + CO2 + Na2CO3. The CO2 gas is the desired product, however the creation of sodium carbonate adds a strong base to the mix. This base must be neutralized with an acidic ingredient to keep neutral (and therefore well flavored) cookies. Baking soda is most commonly used in recipes that also include acidic ingredients such as buttermilk, lemon juice, chocolate, or vinegar. [5] For recipes without these ingredients to neutralize the base produced by the above reaction, baking powder is used. Baking powder is sodium bicarbonate with an acidic solid phosphate mixed in to neutralize the products. Therefore, the reaction occurs to create fluffy, airy cookies without leaving a bitter aftertaste associated with basic foods. Salt Salt may not seem to be an important ingredient, however it should never be omitted to try to make healthier cookies for two main reasons. First, salt is a seasoning that enhances the flavors of everything else. It allows full flavor profiles to be realized and awakens the taste buds. Beyond just making cookies taste better, table salt, or NaCl, plays a vital role in regulating the leavening agent reaction and the production of CO2. NaHCO3 and Na2CO3 are both salts containing polyatomic ions. In aqueous solutions, they separate into their separate ions, Na+, HCO3-, and CO32-. If we include the acid in the original balanced equation, the reaction can be written as NaHCO3 + H+ Na+ + H2O + CO2. Looking at this equation, Na+ appears in the products. According to Le Chatelier’s principle, increasing the amount of product in a reversible reaction pushes the equilibrium towards the left. The balance in Figure 3 represents this concept; increasing the amount of products causes an imbalance and pushes the reaction backwards. Thus, when the sodium ions are in the solution of the batter, they help to slow the forward reaction. Some of the products already exist, so the reaction pace is regulated. Fig 3: Le Chatelier’s principle can be modeled as a balance Source: http:// www.digipac.ca/chemical/mtom/contents /chapter3/chap3_7.htm Chocolate, Nuts, and Conclusion Once all the chemistry has been understood and the correct ratios of each ingredient has been determined so they react correctly together, it is time to get creative with fillers and add your favorites- chocolate, nuts, frosting, and almost anything else! The reasoning behind the precise measuring from grandma’s old family recipes can be much better understood by being familiar with the chemistry behind each ingredient and how they all work together to form cookies. Citations [1] Original Nestle Toll House Chocolate Chip Cookies. [Online]. Available: http://www.verybestbaking.com/recipes/18476/Original-NESTLÉ-TOLL-HOUSEChocolate-Chip-Cookies/detail.aspx. [2] Cole Sugden. (20 December 2012). The Chemistry of Cookies. [Online]. Available: http://prezi.com/jocfizfhq-aj/the-chemistry-of-cookies/. [3] J. H. Czernohorsky and R. Hooker. The Chemistry of Baking. [Online]. Available: http://nzic.org.nz/ChemProcesses/food/6D.pdf. [4] “Butter, Shortening, and Oil: The Fats we Bake With,” in American Baking Essentials. The Prepared Pantry, 2005, ch. 6, pp. 3-6. [5] Amanda Romaniello. (7 July 2009). Everyday Science: Chocolate Chip Cookies Rely on Good Chemistry. [Online]. Available:http://www.underthemicroscope.com/ blog/everyday-science-chocolate-chip-cookies-rely-on-good-chemistry.