Erosion-corrosion of carbon steel in crude oil: velocity effects on the
advertisement

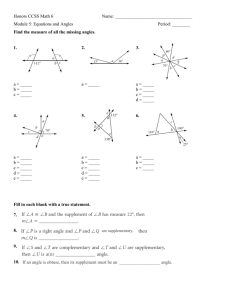
Erosion-corrosion of carbon steel in crude oil: velocity effects on the erosion-corrosion map G. H. Abdulrahman and M. M. Stack Department of Production Engineering Arabian Gulf Oil Company Benghazi-Libya and Department of Mechanical and Aerospace Engineering Strathclyde University, James Weir Building, 75 Montrose St., Glasgow G1 1XJ, UK Email: Ghaith_23@yahoo.com Phone No: 0913529460 margaret.stack@strath.ac.uk 1 Abstract In this work, the effects of erosion-corrosion on carbon steel in two environments, crude oil and crude oil/ 20% water environments containing Al2O3 particles were investigated using an impinging erosion test rig, at two velocities and at a range of impact angles and applied potentials. The results indicated that the corrosion contribution increased with an increase in the percentage of reservoir water. It was observed that the erosion rate changed as a function of impact angle and applied potential and depended on the nature of the corrosion product formed on the surface and the presence of the oil film. Furthermore, increases in velocity altered the erosion-corrosion mechanism significantly. Mechanisms of erosion-corrosion were proposed based on the laboratory studies. The results were used to construct erosion– corrosion maps showing mechanistic interactions, as a function of impact angle and electrochemical potential, at the various exposure velocities. 2 1-Introduction Erosion–corrosion in oil production depends on a range of factors present in downhole oil wells, such as sand concentration, reservoir pressure, temperature, and H2S and CO2 concentration. These conditions may lead to serious mechanical and chemical degradation resulting in economic losses due to maintenance, leaks and replacement of important components [1-4]. Sand production from reservoirs with oil can be controlled by designing a Gravel Pack which prevents sand uptake as the oil is processed from upstream to downstream conditions [5]. In some oilfields, sand content can be controlled by decreasing the velocity of the production through controlling the choke size in the production process [5]. In such conditions, it is important to determine the proportion of water in the environment, the sand content, the operating electrochemical conditions such as potential, and the properties of the particles, such as size, shape, concentration and impact angle. In this paper, the effects of velocity, impact angle and applied potential were assessed for carbon steel in a range of crude oil and oil/water slurries. Erosion-corrosion maps were generated based on the results showing the variation in wastage and regime of degradation as a function of these variables. The potential applications of the maps to such materials issues in petroleum production are described in this paper. 2. Experimental details 2.1 Erosion-corrosion test methods The erosion–corrosion tests were carried out using an impinging jet apparatus is described elsewhere [7]. This consisted of a jet of particles in an aqueous flow enabling the effect of erosion variables to be evaluated independently of each other. The impact angle of the specimen could be varied by rotating the stationary specimen. The slurry was composed of aluminium oxide (Al2O3) 150-300µm in oil and oil/20% 3 water, pH value of oil and mixed environment were 5 and 8.0 at temperature 500 C. The test specimen was connected to an electrochemical cell as shown elsewhere (1). The reference electrode was saturated calomel. Potentiodynamic polarization curves were measured through sweeping the potential in a positive direction from −800 to 800 mV at a sweep rate of 200 mV min-1 during the test. Erosion–corrosion tests were conducted at five applied potentials namely, -400 mV, 0 mV and 400mV (SCE) for 30 minutes using a computer controlled ACM potentiostat. The test material was carbon steel supplied by Kelvin Steel Glasgow with chemical composition as percentage: C: 0.18, Mn 1.6, Si 0.55, Cr 0.25, Cu: 0.35, Ni: 0.3, S: 0.008. The chemical composition of crude oil in ppm (mass) was (Ca: 33.26, Na: 4.26, K: 1.07, H2S: 0.0007). The dimensions of the specimens were 25mm × 10mm × 4 mm. The area exposed to impingement jet was 0.19cm2, whilst the remaining area was covered by a coating in order to ensure that all corrosion measurements related to the erosion-corrosion process only. Mass change measurements were made of the samples post testing using a Metter electronic balance. The tests were carried out at two impact velocities i.e. 2.5 and 4.5 m s−1, for 30 minutes. The reproducibility of the experiments was estimated to be ± 5% calculated between two consecutive experiments. At the end of the test, the samples were cleaned with distilled water to remove any deposited material. Following exposure, the morphology of the eroded specimens was evaluated using scanning electron microscopy (SEM). 3. Results 3.1 Electrochemical monitoring Fig. 2 (a) shows impact velocity 2.5 m s−1 in the crude oil environment. The average free corrosion potential (Ecorr) was between -405mV and -415 mV approximately for all three impact angles and there was a general decrease in the current density at all impact angles compared with oil/water environment Fig. 2 (b). This could be due to the oil film protecting the surface of the specimen from corrosion by inhibiting dissolution of ions of the steel in the hydrocarbon environment (as indicated by the decrease in the current density [8]). 4 Conversely, the percentage of reservoir water in crude oil had a significant effect in enhancing the corrosion. Fig. 3 (a) shows that at an impact velocity 4.5 m s−1 in the crude oil environment containing particles sized150-300µm, the current increased very slowly with increase in impact angle from 15° to 45°, which is consistent with the findings of previous research [8]. Fig. 3 (b) shows impact velocity 4.5 m s−1 in the 20% water with crude oil environment. The current density was increased compared with the previous impact velocity 2.5 m s−1 Fig. 2(b). 3.2. Volume loss and microscopy results The total mass loss kec, corrosion and erosion contribution for carbon steel in the two environments was recorded at impact velocities, 2.5, and 4.5m s−1. Tables 1-4 show the results of calculation of volume loss for erosion contribution (ke), corrosion contribution (kc) and total erosion-corrosion (kec) for different velocities with various impact angles and applied potentials. For the volume loss results, kec represented the measured erosion-corrosion rate. kc was calculated from the Faradic conversion of the current density to mass loss. The value of ke, the erosion contribution, was calculated from the equation: kec= ke + kc (1) In the crude oil environment at impact velocity 2.5 m s−1, Fig. 4-6, the value of corrosion contribution kc was significantly lower than the value of the erosion contribution ke. The erosion-corrosion rate appeared to be independent of impact angle. By contrast in the crude oil and oil/water conditions, Fig. 5-7 the total erosioncorrosion rate appeared to be a maximum at intermediate impact angles of 45o and at potentials of 400mV. In the oil/water environment, the value of the corrosion contribution (kc) was greater than that for erosion (ke). The exception was at 2.5 m s−1 and potential of -400mV, Fig.5 (a). 5 3.3. SEM analysis Figures 8-9 show scanning electron micrographs of eroded carbon steel. The surface morphologies were significantly affected by the impact velocity and impact angle in both crude oil and oil/water environments. For carbon steel in crude oil environment, there was a significant increase in deformation due to particle impact, Fig. 8 (a). The surface of the specimens in the crude oil environment led to less degradation compared to that observed the oil/water conditions, Fig. 9 (a). 4. Discussion 4.1 Trends on the effect of mass change as a function of impact angle and applied potential The results for carbon steel in the two environments indicate that there is a significant reduction in the corrosion current density (Figures 2-3(a-b) when the experimental conditions are changed from oil/water to crude oil environments. This indicates that corrosion contribution is reduced significantly in crude oil environments and this can be attributed to a decrease in the diffusion of iron (Fe) ions in the fully hydrocarbon environment [8]. In addition, the diffusion of oxygen is higher in crude oil than in oil/water and this is the possibly the reason why the cathodic current densities are higher than the anodic current densities in such conditions (Figures 2-3(a). In the oil/water environments, in these conditions, a maximum corrosion current is observed at an impact angle 45o (figures 2-3(b). This indicates that in oil/ water environment, where formation of a corrosion product is facilitated, particle impacts angle at 45o tend to cause higher degradation rates, unlike that which is observed in the oil environment containing particles where the maximum wastage recorded is at 45o and 90o (Figures 2-3(a). 6 In the crude oil and combined environment, Fig. 4-7(a-c), there is a slightly higher mass loss at 400 mV, Fig. 4-7 (c) compared to that observed at lower potentials Fig. 4-7 (a). The trends for the effect of impact angle indicate that the maximum is at 45o and 90o consistent with results on the polarization data, Fig. 2-3(a-b).In the oil/ water environment, there is a clear indication of a maximum erosion-corrosion rate at impact velocity 4.5 m s−1, Fig. 7(a-c). 4.2. Erosion-corrosion maps Erosion-corrosion (kec) maps were constructed to show that the transition between wastage regimes i.e. with the low value defined as less or equal to 6 mg cm −2 h−1 , medium between 6 and 50 mg cm −2 h−1 and high greater or equal to 50 mg cm −2 h−1 (1-4). Figures 10 (a-b) show carbon steel at constant impact velocity of 2.5 m s−1 in the crude oil and mixed environment. It is interesting to see that the low wastage regimes dominate at all impact angles and applied potentials, and there is no evidence of the presence of a medium wastage regime on the map. For carbon steel in the crude oil environment at impact velocity 4.5 m s−1 Fig. 11 (a), it can be seen that the low wastage regime dominates and there is no evidence of medium wastage on the specimen with increase in impact velocity. Fig. 11 (b) shows that in the combined environment, the medium wastage regime predominates. This is attributed to the relatively high value of the corrosion contribution kc at 4.5 m s−1. Erosion-corrosion additive-synergism maps for carbon steel in the crude oil and oil/water environments containing particles have been constructed. Tables 1-4 show the values of Δke /Δkc ratio for the carbon steel in the different environments. They show the differences between the boundaries as a function of impact angle and applied potential. For the fully hydrocarbon conditions, Fig. 12(a), it is interesting to see that the synergistic effect has a significant effect on the specimen at 2.5 m s−1 . On the other hand, Fig.12(b), shows carbon steel in the combined environments. It is clear that the percentage of synergistic effect on the specimen is decreased compared 7 with that observed in the crude oil environment Fig. 12(a), while the additivesynergistic regime becomes dominant across the entire whole map. This is attributed to the presence of the passive film in such conditions. From this map, it is clear that both erosion and corrosion enhance each other over this window of conditions. Fig. 13(a) shows carbon steel in the crude oil environment at 4.5 m s−1. It can be seen that the synergistic effect dominates the entirety of the map. However, Fig. 13(b ), in the crude oil/20% water environment, it can be seen that the boundaries change to additive-synergistic in these conditions. Erosion-corrosion mechanism maps have been constructed for carbon steel in crude oil and oil/water environments. Fig. 14(a), shows at constant impact velocity of 2.5 m s−1 for carbon steel in the crude oil environment. It is observed that erosion– passivation dominates in all areas between impact angles 15° - 90° and between applied potentials -400mV to 400mV. However, in the crude oil/20% water environment, the passivation-erosion regime occupies the area in anodic conditions, which can be attributed to the passive film forming on the surface of the specimen. At impact velocities of 4.5 m s−1 Fig.15 (a), it is interesting to observe that the erosionpassivation regime predominates for the fully hydrocarbon environment. In oil/20% water at similar velocities, Fig. 15 (b), it is clear that passivation–erosion regime predominates. Also, there is no evidence of the presence of erosion-passivation effect on the map with increasing in impact velocity from 2.5 m s−1 to 4.5 m s−1 , indicating that passivation has a more significant effect on the erosion-corrosion process, in these conditions, as evidenced also by the polarization and volume loss data above. Clearly, erosion-corrosion maps are important tools which can be used to predict wastage in such conditions. The results on the effect of velocity indicate a significant change depending on the conditions. Further work will be to assess the boundaries of such maps, over a wider range of variables for oil and gas processing, such that a materials and process parameter map be constructed for such environments. 8 5. Conclusions (i) (ii) (iii) The effects of impact angle and electrochemical potential on the erosion–corrosion of carbon steel have been assessed in crude oil and oil/water environments containing particle sizes ranging from 150 300 m . Erosion-corrosion wastage, additive-synergism and mechanism maps have been constructed based on the results. The results have indicated that the erosion-corrosion regimes are highly dependent on the exposure conditions, with the enhanced passivation in the water containing environments, resulting in either an increase and reduction in the overall wastage, depending on the exposure conditions. Potentiostat RE Ejector Body Inlet Nozzle W.E A.E Exit Nozzle Test Specimen Solution Fig.1: Electrochemical connection in the slurry erosion test. 9 800 15° 600 600 400 200 Potential (mV) Potential (mV) 800 90° 45° 0 -200 45° 90° 400 15° 200 0 -200 -400 -400 -600 -600 -800 -800 0.0001 0.01 0.001 0.1 0.0001 -2 0.01 0.1 1 -2 Current (mA cm ) Current (mA cm ) (a) (b) Figure (2) Polarization curves for carbon steel at various impact angles in (a) crude oil and (b) crude oil/ 20% water at impact velocity 2.5 m s−1. 800 800 45° 600 600 400 200 Potential (mV) Potential (mV) 90° 15° 0 -200 400 0 -600 -600 0.001 0.01 0.1 15° -200 -400 0.0001 90° 200 -400 -800 45° -800 -2 Current (mA cm ) 0.0001 0.01 0.1 -2 Current (mA cm ) 10 1 (a) (b) 2.2 -1 1.8 Ke Kc 2.4 Kec 2.0 Ke Kc Kec 2.2 2.0 -1 -2 1.6 1.4 1.2 1.0 0.8 0.6 Volume loss .mg cm .h -2 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 Ke Kc Kec Volume loss .mg cm .h -2 Volume loss .mg cm .h -1 Figure (3) Polarization curves for carbon steel at various impact angles in (a) crude oil and (b) crude oil/ 20% water at impact velocity 4.5 m s−1. 1.8 1.6 1.4 1.2 1.0 0.8 0.6 0.4 0.4 15° 45° 15° 90° 45° 90° 15° Impact angle Impact angle (a) 45° 90° Impact angle (b) (c) Fig.4: Volume loss as function of impact angle for carbon steel in crude oil at 2.5 m s−1impact velocity (a) -400mV (b) 0mV (c) 400mV Ke Kc Kec 2.6 -2 2.0 1.8 1.6 1.4 1.2 -1 2.2 2.0 1.8 1.6 1.4 1.2 1.0 45° 90° Impact angle 2.5 2.0 1.5 1.0 1.0 15° 0.8 15° 45° 90° Impact angle (a) Ke Kc Kec 3.5 3.0 -2 -1 2.4 Volume loss .mg cm .h -2 Volume loss .mg cm .h -1 2.4 2.2 Ke Kc Kec 2.8 Volume loss .mg cm .h 2.6 (b) 15° 45° Impact angle (c) Fig.5: Volume loss as function of impact angle for carbon steel in oil /20% water at 2.5 m s−1impact velocity (a) -400mV (b) 0mV (c) 400mV 11 90° 6.0 Kc 5.5 5.5 5.0 5.0 Kec 3 2 -1 4.5 -2 -2 4 Volume loss .mg cm .h Volume loss .mg cm .h -1 -1 5 Ke 6.0 4.0 3.5 3.0 2.5 2.0 1.5 1 Volume loss .mg cm .h Ke Kc Kec 1.0 15° 45° 90° 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 15° Impact angle 45° 90° 15° Impact angle (a) 45° 90° Impact angle (b) (c) Fig.6: Volume loss as function of impact angle for carbon steel in crude oil at 4.5 m s−1impact velocity (a) -400mV (b) 0mV (c) 400mV Ke Kc Kec6.5 6.0 5.5 6.0 3.0 2.5 2.0 -2 5.0 4.5 4.0 3.5 3.0 2.5 1.5 15° 45° 90° 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.0 15° Volume loss .mg cm .h 3.5 6.5 6.0 5.5 Volume loss .mg cm .h 4.0 7.0 -1 -1 4.5 -2 Volume loss .mg cm .h -1 5.0 -2 Ke Kc Kec 45° 90° 2.0 Impact angle Impact angle (a) (b) 15° 45° Impact angle (c) Fig.7: Volume loss as function of impact angle for carbon steel in oil /20% water at 4.5 m s−1impact velocity (a) -400mV (b) 0mV (c) 400m 12 90° (a) (b) (c) 13 (d) Fig.8: Scanning electron micrographs of eroded carbon steel test specimen at 4.5 m s−1, impact angle 90° (a) in crude oil (b) analysis of the spectrum-1 (c) analysis of the spectrum-2 (d) analysis of the spectrum-3 (a) (b) (c) 14 (d) Fig.9: Scanning electron micrographs of eroded carbon steel test specimen at 4.5 m s−1, impact angle 90° in (a) oil /20% water (b) analysis of the spectrum-1 (c) analysis of the spectrum-2(d) analysis of the spectrum-3 90 Impact angle Impact angle Low Wastage 45 90 45 Low Wastage 15 15 -500 -400 -500 400 0 E(mV) -400 (a) 400 0 E(mV) (b) Figure (10): Erosion-corrosion wastage maps for carbon steel in (a) crude oil (b) crude oil / 20% of water at 2.5 m s−1 Low Wastage 45 45 15 15 -500 90 Low Wastage 90 Impact angle Impact angle Medium Wastage -400 0 E(mV) 400 -500 (a) -400 0 E(mV) (b) 15 400 Fig.11: Erosion-corrosion wastage maps for carbon steel at 4.5 m s−in (a) crude oil (b) oil /20% water 90 Impact angle Impact angle 90 Synerg istic Dominated Synergistic Dominated 45 45 Additive-Synergistic Additive-Synergistic 15 15 Synergistic Additive -500 -400 -500 400 0 0 E (mV) -400 E(mV) (a) 400 (b) 90 90 Impact angle Impact angle Fig.12: Erosion-corrosion additive-synergism maps for carbon steel at 2.5m s−1 in (a) crude oil (b) oil /20% water. 45 45 Additive-Synergistic Synergistic Dominated 15 15 Additive-Synergistic Additive -500 -400 0 -500 400 E(mV) 16 -400 0 E (mV) 400 (a) (b) Fig.13: Erosion-corrosion additive-synergism maps for carbon steel at 4.5m s−1 in (a) crude oil (b) oil /20% water. 45 15 Erosion-Passivation 90 Impact angle Impact angle Erosion-Passivation 90 45 Passivation-Erosion 15 -500 -400 0 E(mV) 400 -500 -400 0 E(mV) 400 90 Impact angle Impact angle (a) (b) −1 Fig.14: Erosion-corrosion mechanism maps for carbon steel at 2.5 m s in (a) crude oil (b) oil /20% water. 45 Erosion-Passivation 45 Passivation-Erosion Passivation-Erosion 15 -500 90 15 -400 0 E(mV) 400 -500 -400 0 E(mV) (a) (b) Fig.15: Erosion-corrosion mechanism maps for carbon steel at 4.5 m s−1 in (a) crude oil (b) oil /20% water. 17 400 Tables.1:Volume loss as function of impact angles for carbon steel in crude oil at 2.5 m s−1 (a) -400mV (b) 0mV (c) 400mV. (a) Impact angle 15° 45° 90° Ke(mg cm −2 h−1) Kc(mg cm −2 h−1) Kec(mg cm −2 h−1) Ke/Kc Δke/Δkc 1.429 1.658 1.059 3.21E-01 3.42E-01 5.91E-01 1.75 2 1.65 4.4517 4.8479 1.7918 4.177 4.54 1.676 (b) Impact angle 15° 45° 90° Ke(mg cm −2 h−1) Kc(mg cm −2 h−1) Kec(mg cm −2 h−1) Ke/Kc Δke/Δkc 1.455 1.77 1.44 3.95E-01 3.40E-01 4.10E-01 1.85 2.11 1.85 3.68 5.20 3.512 4.63 4 5.23 4.837 (c) Impact angle 15° 45° 90° Ke(mg cm −2 h−1) Kc(mg cm −2 h−1) Kec(mg cm −2 h−1) Ke/Kc Δke/Δkc 1.556 1.947 1.73 4.24E-01 3.57E-01 4.70E-01 1.98 2.304 2.2 3.669 5.45 3.68 4.376 6.107 4.836 Tables.2:Volume loss as function of impact angle for carbon steel in oil /20% water at 2.5 m s−1 impact velocity (a) -400mV (b) 0mV (c) 400mV. (a) Impact Ke(mg Kc(mg Kec(mg angle Ke/Kc cm −2 h−1) cm −2 h−1) cm −2 h−1) Δke/Δkc 15° 1.11E+00 1.189 2.3 1.070 3.55 4 45° 1.21E+00 1.289 2.5 1.0644 0.91 90° 1.03E+00 1.399 2.43 1.3567 2.76 (b) Impact angle 15° 45° 90° Ke(mg cm −2 h−1) Kc(mg cm −2 h−1) Kec(mg cm −2 h−1) Ke/Kc Δke/Δkc 1.089 0.949 0.849 1.51E+00 1.75E+00 1.65E+00 2.6 2.7 2.5 0.72 8 0.54 0.514 0.88 0.209 0.527 (c) 18 Impact angle 15° 45° 90° Ke(mg cm −2 h−1) 0.979 1.549 0.819 Kc(mg cm −2 h−1) 1.77E+00 1.85E+00 1.83E+00 Kec(mg cm −2 h−1) Ke/Kc Δke/Δkc 2.75 3.4 2.65 0.55 0.836 0.447 0.483 0.725 0.398 Table.3: Volume loss as function of impact angles for carbon steel in crude oil 4.5 m s−1impact velocity (a) - 400mV (b) 0mV (c) 400mV (a) Impact Ke(mg Kc(mg Kec(mg angle Ke/Kc cm −2 h−1) cm −2 h−1) cm −2 h−1) Δke/Δkc 15° 7.50E-01 4.25 5 5.66 6.0 45° 8.00E-01 4.62 5.42 5.77 5.816 90° 8.23E-01 3.827 4.65 4.65 5.889 (b) Impact angle 15° 45° 90° Ke(mg cm −2 h−1) Kc(mg cm −2 h−1) Kec(mg cm −2 h−1) Ke/Kc Δke/Δkc 4.647 4.859 4.332 8.53E-01 8.91E-01 8.68E-01 5.5 5.75 5.2 5.449 5.45 4.99 7.33 5.88 6.46 (c) Impact angle 15° 45° 90° Ke(mg cm −2 h−1) Kc(mg cm −2 h−1) Kec(mg cm −2 h−1) Ke/Kc Δke/Δkc 4.55 4.9 4.2 9.00E-01 1.00E+00 1.20E+00 5.45 5.9 5.4 5.055 4.9 3.5 7.5 5.26 4.08 Table.4: Volume loss as function of impact angles for carbon steel in oil /20% water at 4.5 m s−1impact velocity and particle size 150 300 m (a) - 400mV (b) 0mV (c) 400mV (a) Impact Ke(mg Kc(mg Kec(mg angle Ke/Kc cm −2 h−1) cm −2 h−1) cm −2 h−1) Δke/Δkc 15° 3.37E+00 2.53 5.9 0.75 0.57 45° 3.60E+00 2.4 6 0.66 0.29 90° 3.52E+00 1.98 5.5 0.56 0.42 (b) Impact angle 15° 45° 90° Ke(mg cm −2 h−1) 2.4 2.05 1.9 Kc(mg cm −2 h−1) 4.00E+00 4.45E+00 4.30E+00 Kec(mg cm −2 h−1) Ke/Kc Δke/Δkc 6.4 6.5 6.2 0.6 0.46 0.44 0.37 0.107 0.3 (c) 19 Impact angle 15° 45° 90° Ke(mg cm −2 h−1) 2.42 2.23 2.08 Kc(mg cm −2 h−1) 4.23E+00 4.55E+00 4.42E+00 Kec(mg cm −2 h−1) Ke/Kc Δke/Δkc 6.65 6.78 6.5 0.57 0.49 0.47 0.457 0.191 0.446 References [1] M. M. Stack, T. M. Abd El Badia, on the construction of erosion-corrosion maps for wc/co-cr-based coatings in aqueous conditions, Wear 261 (11-12) (2006)1181– 1190. [2] M. Stack, G. Abdulrahman, Mapping erosion-corrosion of carbon steel in oil exploration conditions: Some new approaches to characterizing mechanisms and Synergies, Tribology International, 43 (7) (2010) 1268-1277. [3] M. M. Stack, S. Zhou and R.C. Newman, Characterization of synergistic effects between erosion and corrosion in an aqueous environment using electrochemical techniques. Corrosion Science, 52 (1996) 934-946. [4] M.M .Stack and Pungwiwat N, Erosion–corrosion mapping of Fe in aqueous slurries: some views on a new rationale for defining the erosion–corrosion interaction, Wear 256 (5 ) (2004) 565-576. [5] R .Hamzah, D.J Stephenson and J.E. Strutt, Erosion of material used in petroleum production, Wear 186 (187) (1995) 493-496. [6] Yu .Fang and Guoan Zhang, Mechanical properties of CO2 corrosion product scales and their relationship to corrosion rates, Corrosion Science 50 (2008) 2796– 2803. [7] J.B. Zu, I.M. Hutchings and G.T. Burstein, Design of a slurry erosion test rig, Wear140 (2) (1990)331–344. [8] B.R Tian and Y.F. Cheng electrochemical corrosion behaviour of X-65 steel in the simulated oil sand slurry. I. Effects of hydrodynamic condition, Corrosion Science 50 (2008) 773-779. [9] G.T .Burstein, Y. Li and I.M. Hutchings, The influence of corrosion on the erosion of aluminium by aqueous silica slurries, Wear 186-187 (Part 2) (1995) 515 – 522. 20 21