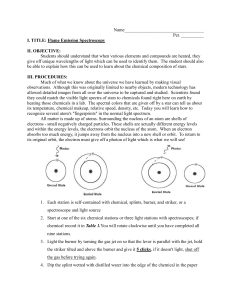
Flame test observations
advertisement

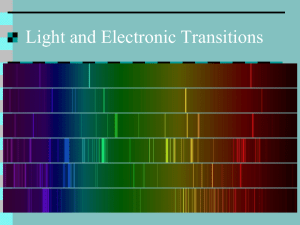
FLAME TESTS as Evidence of ATOMIC STRUCTURE INTRODUCTION Just as a fingerprint is unique to each person, the color of light emitted by metals heated in a flame is unique to each metal. In this activity, the characteristic color of light emitted for several metals will be observed. PURPOSE To observe the atomic spectra of a variety of metal ions and to identify the ion present in an unknown solution. EQUIPMENT AND MATERIALS 1. Chemical splash goggles – required 2. aprons, and gloves 3. unknown(s) – beaker containing unknown solution and wooden splints EQUIPMENT AND MATERIALS – each of six stations 4. one labeled (name and formula) 250 mL beaker half filled with one of the following solutions AND wooden splints NaCl, KCl, LiCl, CaCl2, SrCl2, CuCl2, and unknown(s). 5. If available – place one or more pieces of blue cobalt glass at the station with the KCl – potassium chloride 6. Laboratory burner and device to light burner. 7. Colored drawing pencils PROCEDURE Safety note: Wear approved chemical splash goggles and – if required by your teacher, a chemical-resistant apron and gloves. The metal salts used allow the burned splints to be safely disposed of in the trash, but the teacher, to avoid any possibility of a trashcan fire, should do this. 1. Start a new entry in your science notebook. Include title, date, and purpose. 2. Make a data table for your observations. Flame test observations Solution Metal ion present Colored sketch of flame Written description of color(s) observed and intensity (bright/faint) of flame NaCl KCl LiCl CaCl2 SrCl2 Cucl2 Unknown (code: ) 3. Put on safety equipment (chemical splash goggles required; your teacher may also ask you to wear a safety apron and/or gloves) 4. Go to your assigned station. Note the name and formula of the metal ion solution that you are testing. 5. Carefully light laboratory burner. 6. Obtain a wooden splint from the solution and slowly pass the wooden splint back and forth through the flame. Pass the splint through the flame at its hottest point – just above the inner blue cone. Do not leave the splint in the flame long enough for it to burn... keep the splint moving! You may redip the wooden splint in the solution and redo the test several times. Return the wood splint to the solution when you are done testing. [If the splint actually burns, leaving a black area, give the burned splint to your teacher for proper disposal.] 7. Record your observations - the color and intensity (bright/faint) of the flame – in your data table. The color given off by the salt is the initial color observed, not the yellow-orange color produced by the burning wood. 8. Move to the next station – when directed by your teacher – and repeat steps 1–4 with another salt, until all ions have been tested. 9. Follow your teacher’s instructions for testing your unknown(s). Be sure to record which unknown you were assigned. Record your observations in your data table and determine which ion is present in this solution. 10. Once the laboratory work is complete, wash hands with soap or detergent. 11. Perform calculations and answer all analysis questions. CONCLUSIONS: 1. Make a claim about the identity of your unknown and support it with evidence from this lab. 2. Read the information provided in the ‘EXPLANATION’ section on the next page to learn how the color of the flame is produced. Use this information to answer the following questions: a. What happens on a subatomic level to produce the color of the flame? a. Why do different chemicals emit different colors of light? b. Why do the chemicals have to be heated in the flame first before the colored light is emitted? 3. Discuss some of the limitations of using flame tests to identify ions present in solution. 4. Propose at least one possible method for improving the accuracy of results in this laboratory experiment. 5. Do you think we can use the flame test to determine the identity of unknowns in a mixture? Why or why not? CONNECTIONS: The electromagnetic spectrum is shown below. Recall that energy is proportional to frequency, while frequency is inversely proportional to wavelength. Use this information to answer questions 1-4 below. 6. List the colors observed in this lab from the highest energy to the lowest energy. 7. List the colors observed in this lab from the longest wavelength to the shortest wavelength. 8. What is the relationship between energy and wavelength? 9. Explain why we did not see distinct lines (like on an emission spectrum) when the metal salts were burned. APPLICATIONS: 10. Colorful light emissions are applicable to everyday life. Where else have you observed colorful light emissions? Are these light emission applications related? Explain. 11. Some fireplace logs (commercially made) burn with a red and/or green flame. Using the information in this experiment, what elements could be responsible for these colored flames? 12. At a 4th of July celebration, aerial fireworks produce a variety of colors. Which element(s) would you include to produce crimson red? Yellow? 13. An arson investigator is called to a suspious fire that destroyed a house. The arson investigator collects a sample of debris, which was found at the base of the structure, containing some accelerant used to start the fire. Once the investigator goes back to the lab and tests the sample, a purple flame is emitted from the sample. What metal was part of the accelerant used to burn the house? 14. A forensic scientist does a flame on a substance that was found at a crime scene. What might the scientist conclude if the flame turns green? EXTENSIONS: 15. Research some information about the origin of fireworks. (The websites listed at the beginning of this activity might be helpful). Explain how they are made, what chemicals are used, what color they burn and their uses. CALCULATIONS: 16. A green line of wavelength 486 nm is observed in the emission spectrum of hydrogen. Calculate the energy of one photon of this green light. Use the mathematical relationships ν = c/λ and E = hν, where ν = frequency, c = speed of light (3.00 x 108 m/s), λ = wavelength, h = Plank’s constant (6.63 x 10-34 J·s), and E = energy. ----------EXPLANATION – How are the colors of a flame test made? – The Basics A flame test is a procedure used to test qualitatively for the presence of certain metals in chemical compounds. When the compound to be studied is excited by heating it in a flame, the metal ions will begin to emit light. Based on the emission spectrum of the element, the compound will turn the flame a characteristic color. Many elements produce colors in the flame when heated. The origin of this phenomenon lies in the arrangement, or “configuration” of the electrons in the atoms of the different elements. In the “solar system” model of the atom first proposed by Ernest Rutherford and Niels Bohr in the early 1900s, the electrons were pictured as moving around the nucleus in circular orbits in a similar manner that the planets in our solar system orbit the sun. As envisioned by Bohr, during heating, one or more electrons may absorb energy in sufficient amounts to “jump” to an orbit farther away from the nucleus. Since the electron has a higher potential energy in its new orbit, the electron is said to be in a higher energy level. When the electron has been promoted to a higher energy level, the atom is said to be in an excited state. When the electrons drop from a higher energy level to a lower energy level (in an orbit closer to the nucleus), energy is released. In the flame test, if this energy has the form of visible light, the flame will produce a color characteristic of the element. Different elements have a unique color in its flame which can be used to identify an element. When the electrons are in their lowest energy orbits (closest to the nucleus), the atom is said to be in its ground state. EXPLANATION – How are the colors of a flame test made? – More Detail When a substance is heated in a flame, the atoms absorb energy from the flame. This absorbed energy allows the electrons to be promoted to excited energy levels. From these excited energy levels, there is a natural tendency for the electrons to make a transition or drop back down to the ground state. When an electron makes a transition from a higher energy level to a lower energy level, a particle of light called a photon is emitted (see Figure 1). Both the absorption and emission of energy are quantized – only certain energy levels are allowed. An electron may drop all the way back down to the ground state in a single step, emitting a photon in the process. Alternatively, an electron may drop back down to the ground state in a series of smaller steps, emitting a photon with each step. In either case, the energy of each emitted photon is equal to the difference in energy between the excited states and the state to which the electron relaxes. The energy of the emitted photon determines the color of light observed in the flame. The flame color may be described in terms of its wavelength, as shown in the chart below: color Violet Blue Blue-green Green Yellow-green Yellow Orange Red Wavelength Region (nm) 400-425 425-480 480-500 500-560 560-580 580-585 585-650 650-700 The color of light observed when a substance is heated in a flame varies from one substance to another. Because each element has a different spacing of electron energy levels, the possible electron transitions for a given substance are unique. Therefore, the difference in energy between energy levels, the exact energy of the emitted photon, and the corresponding wavelength and color are unique to each substance. As a result, the colors observed when a substance is heated in a flame may be used as a means of identification. You may recall studying the emission spectra of elements earlier in this course, when we studied the electromagnetic spectrum. We observed the emission spectrum of the hydrogen using a discharge tube, a slim tube containing hydrogen gas at low pressure with an electrode at each end. If you put a high voltage across this (say, 5000 volts), the tube lights up with a bright pink glow. If the light is passed through a prism or diffraction grating, it is split into its various colors. What you would see is a small part of the hydrogen emission spectrum. Most of the spectrum is invisible to the eye because it is either in the infra-red or the ultra-violet. The photograph shows part of a hydrogen discharge tube on the left, and the three most easily seen lines in the visible part of the spectrum on the right. http://www.chemguide.co.uk/ato ms/properties/hspectrum.html