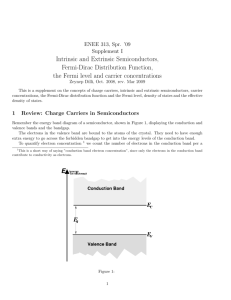
Fermi energy in intrinsic semiconductor
advertisement

Lecture 13: After the band theory and band structures where do we go from here? Flat Band Diagrams simplified representation for energy However, some functions cannot be seen, such as direct or indirect band gap. Insulators (Diamond): Each s-band containing N atoms has space for 2N electrons. If the highest s- band is occupied by 2 electrons, thus the band is completely filled (insulator) when you apply an electric field, electrons cannot absorb energy and they can’t drift. 1 Alkali Metals (one valence electron per atom): The valence band is half- filled. There are available states for electrons. Application of an electric field causes electron drift. 2 Bivalent Metals: They should be insulators, but they are not, why? Because the upper band partially overlap (look to the diagram) and electrons flow in the lower portion of the next higher band (almost partially filled bands). Conductors Intrinsic Semiconductors: Valence bands are filled. They have a narrow forbidden gap. Energy can excite electrons from the completely filled valence band into the empty conduction band. 3 Semiconductors: Valence and conduction bands consist of mixed (hybrid) S- and p- states 8 highest states (2s, 6p) split into two separate (s+p) bands. 4 As you see from the graph each band consists o one s-state and 3 p-states. The valence band can have 4 electrons. The conduction band can have 4 electrons Intrinsic semiconductor (Si, Ge) have 4 valence electrons ∴ The valence bands are completely filled and the conduction bands are empty. There is no gap bet? The 1-s state and the 3-p states in the valence band. Important Note: All covalent band elements have the same bands shown in the figure. The energy gap causes the difference 5 The loser atomic number⟶ highest energy gap in IV elements Fermi energy in intrinsic semiconductor: Intrinsic semiconductor: 6 Fermi energy is defined as the energy where Fermi distribution function F(E) equals ½. In Intrinsic semiconductor: At T=0 F(E) = 1 for E < Ev F(E) = 0 for E > Ec 7 At higher temperatures Some of the electrons have been excited into the conduction band. F(E) is slightly reduced at the top of valence band F(E) is not zero at the bottom of the conduction band. 8 EF is the center of the gap. 9 Next, let’s evaluate the electrical conductivity: Metals: In classical theory, we assumed that all electrons drift under the influence of an electric field. All electrons contribute to conductivity. Is it right? Lets investigate that from the following diagram for electron velocities (a) equilibrium (b) when an electric field is applied DRAWING Only electrons near the Fermi energy contribute to the conductivity Derivation of Conductivity: Current density j j = N e vf n*: numer of electrons displaced by electric field vf : Fermi velocity (velocity at Fermi surface) 10 The effect of an applied electric field: Increase ∆𝐸 at the Fermi energy to higher levels DRAWING n*= N(Ef )∆E j = evf N(Ef )∆E dE = evf N(Ef ) ∙ dK dK Let me find 𝑑𝐸 𝑑𝐾 h2 k 2 E= for free electrons 2m dE h2 k h2 P hmvf = = = = hvf dk 2m mh m j = evf 2 N(Ef )h dk Let’s find a relevant expression for dk ℇ = electric field dv F=m dt 11 𝑒ℇ = 𝑑𝑝 𝑑𝑘 =ℎ 𝑑𝑡 𝑑𝑡 eℇ dk = dt h 2 2 ( ) j = e vf N Ef ℇζ where ζ = dt (the relaxation time: time between collisions) j = e2 vf 2 ζ N(Ef )ℰ For 3 dimensions: 1 j = e2 vf 2 ζN(Ef )ℰ 3 1 2 2 j = e vf ζN(Ef )ℰ 3 Depends on Fermi velocity Occipital density of states at the Fermi level Relaxation time Not all free electrons are responsible for conduction DRAWING -monovalent metals (Cu, Ag, Au): have partially filled valence bands, population density is high near Fermi energy 12 → high conductivity -Bivalent metals: overlap of the upper band electron population near Fermi energy is small → less conductivity 13
![Semiconductor Theory and LEDs []](http://s2.studylib.net/store/data/005344282_1-002e940341a06a118163153cc1e4e06f-300x300.png)