Supplemental Digital Content 1
advertisement

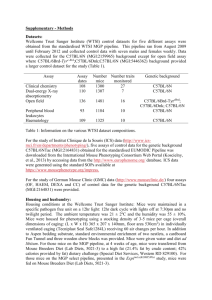
Supplemental Digital Content 1 MATERIALS AND METHODS Animals Six-to-twelve-week old male C57BL/6 and BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions, according to institutional guidelines. Protocols were approved by the Johns Hopkins University Animal Care and Use Committee. Isolation and Expansion of Adult Mouse Kidney-Derived Mesenchymal Stromal Cells KSCs were generated as previously described (1). Briefly, adult C57BL/6 mouse kidneys were first perfused in situ with PBS to remove contaminating blood cells, then harvested and removed of vessels and connective tissue. Kidneys were then minced, partially digested with collagenase IV (Sigma, St. Louis, MO), and placed as explants on fibronectin-coated plates. After several days, mixed cell types arose from adherent tissue explants that were further harvested and seeded on poly-D-lysine–coated plates (Sigma) at 4 x 104 cells/ml in medium containing Iscove's Modified Dulbecco's Medium (IMDM), 20% FBS, 2-mercaptoethanol, L-glutamine, and penicillin-streptomycin (PSN). Several days later, cells remaining adherent to the plates were discarded to minimize contamination with other cells (e.g., fibroblasts, epithelial cells), whereas detached floating kidney spheres were plated on regular flasks and expanded as monolayers. KSCs within 20 passages were used for experiments, with flow cytometric analysis of surface molecules performed as a quality control measure. Bone Marrow-Derived DC Culture BM-derived DCs were generated as described previously (2). Briefly, BM cells from C57BL/6 and BALB/c mice were cultured for 5 days in RPMI 1640 with 10% FBS, L-glutamine, 2mercaptoethanol, PSN, mouse GM-CSF (500 U/ml), and mouse IL-4 (500 U/ml). KSCs were treated with mitomycin (50 µg/ml) at 37°C for 30 min to inhibit their proliferation, washed, and added into initial DC culture at day 0 (KSC:DC at 1:10 ratio). Half of the supernatant was replaced with fresh cytokine-containing medium every other day. DCs were identified as CD11c+ cells, and activation/maturation (MHC class II, CD80) analyzed by flow cytometry. DC-Stimulated T Cell Responses Magnetic bead separation (Miltenyi Biotec, Auburn, CA) was used to isolate CD11c+ DCs from KSC:DC co-cultures and CD4+ T cells from mouse spleen and LNs. Purity of isolated fractions was consistently approximately 90% for DCs and >95% for CD4+ T cells. Isolated BALB/c mouse derived-DCs were co-cultured with CFSE-labeled C57BL/6 mouse-derived CD4+ T cells (allogeneic response) at a ratio of 1:1 for 4 days. T cell proliferation was defined as percentage of cells exhibiting CFSE fluorescence dilution. DC-Stimulated B Cell Responses BALB/c mice were immunized by intraperitoneal injection of 1x107 C57BL/6 mitomycin-treated splenocytes (twice; at day 0 and day 21), and sacrificed at day 24. Bone marrow-derived DCs were generated from C57BL/6 mice, with or without the co-culture of KSCs (C57BL/6). DCs were then purified using anti-CD11c microbeads and co-cultured with the sensitized BALB/c splenocytes (ratio 1:1) for 3 days to stimulate B cell maturation/activation and antibody production. CD19+IgM+ mature B cells were analyzed by flow cytometry. IgG/IgM levels in the supernatants were measured by ELISA (Immunology Consultants Laboratory, Newberg, OR). Islet Transplantation Mouse islet transplantation was performed as previously described (3). Islet preparations were generated from eight-to-twelve-week old BALB/c mice. After clamping the pancreatic duct at duodenal insertion, 3 ml of digestion solution containing 0.5 mg/ml Liberase TL and 0.1 mg/ml DNAse I (Roche Applied Science) in HBSS were injected through the bile duct to distend the pancreas. Pancreata were removed and digested at 37C for 21 min. Crude islets were separated from digested pancreas by density gradient centrifugation (1.098, 1.077, 1.036; Sigma). Islets were further hand picked under a dissecting microscope and stained with dithizone to access the purity (>95%). Eight-week old C57BL/6 mice were then made diabetic (>350 mg/dl on two consecutive measurements) by intraperitoneal injection with a single dose of streptozocin (200 mg/kg). Five hundred BALB/c islets were transplanted under the left kidney capsule of diabetic recipients to achieve normoglycemia (<150 mg/dl) within 24 hours. DCs (5x105) from different treatment groups were co-transferred under the kidney capsule. Blood glucose was monitored to determine the time of rejection, which was defined as the first of three consecutive days of hyperglycemia (>150 mg/dl). In separate experiment, recipients were sacrificed 10 days after transplant. Spleen and draining lymph node (left renal lymph node) were harvested to analyze CD4+CD25+FOXP3+ regulatory T cell populations by intracellular staining and flow cytometry. Splenocytes were stimulated for four hours with PMA (5 ng/ml; Sigma) and ionomycin (500 ng/ml; Sigma) in the presence of GolgiPlug (BD Bioscience) to analyze TNFα- and INFγ- producing T cells by intracellular staining and flow cytometry. Flow Cytometric Analysis Cells were subjected to flow cytometric analysis using a FACScalibur flow cytometer (Becton Dickinson, Mansfield, MA). Data were analyzed using the FCS Express analysis software (De Novo, Los Angeles, CA). Fluorochrome-conjugated antibodies against the following mouse cell surface antigens (as well as relevant isotype controls) were purchased from BD Pharmingen (San Diego, CA) and eBioscience (Los Angeles, CA), unless specifically mentioned: CD3e (145– 2C11), CD4 (L3T4; RM4-5), CD11c (Integrin ax chain; HL3), CD19 (1D3), CD25 (PC61.5), CD80 (B7–1; 16-10-A1), MHC class II (I-A/I-E; M5/114.15.2), Foxp3 (FJK-16s), IgM (µ chain specific; Jackson Immunoresearch Laboratories, West Grove, PA). Statistical Analysis Data are presented as mean ± SD. Results shown are representative of at least three independent experiments. Statistical comparisons between groups were performed by student’s t test, ANOVA tests, and Kaplan-Meier survival curves with log rank testing where appropriate. P< 0.05 was deemed significant. 1. 2. 3. Huang Y, Johnston P, Zhang B, et al. Kidney-derived stromal cells modulate dendritic and T cell responses. J Am Soc Nephrol 2009; 20 (4): 831. Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colonystimulating factor. J Exp Med 1992; 176 (6): 1693. Fiorina P, Jurewicz M, Vergani A, et al. Phenotypic and functional differences between wild-type and CCR2-/- dendritic cells: implications for islet transplantation. Transplantation 2008; 85 (7): 1030.