Supplementary Table 1 (docx 19K)
advertisement
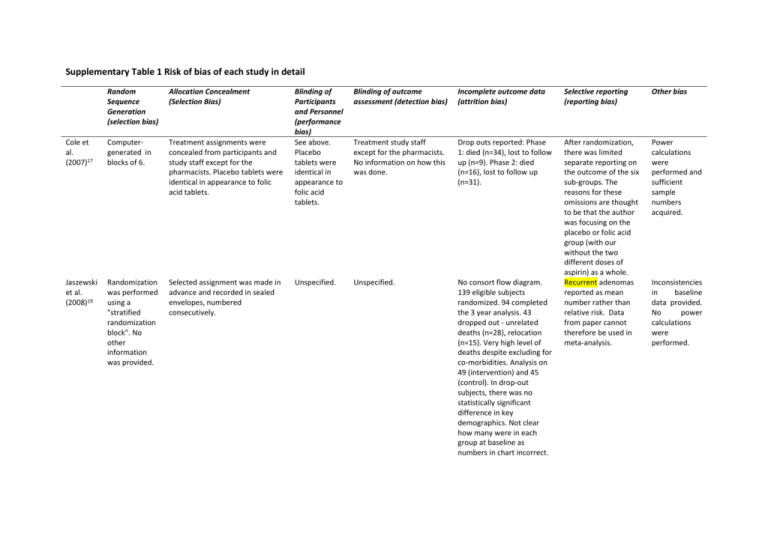
Supplementary Table 1 Risk of bias of each study in detail Random Sequence Generation (selection bias) Allocation Concealment (Selection Bias) Cole et al. (2007)17 Computergenerated in blocks of 6. Treatment assignments were concealed from participants and study staff except for the pharmacists. Placebo tablets were identical in appearance to folic acid tablets. Jaszewski et al. (2008)19 Randomization was performed using a "stratified randomization block". No other information was provided. Selected assignment was made in advance and recorded in sealed envelopes, numbered consecutively. Blinding of Participants and Personnel (performance bias) See above. Placebo tablets were identical in appearance to folic acid tablets. Blinding of outcome assessment (detection bias) Incomplete outcome data (attrition bias) Selective reporting (reporting bias) Other bias Treatment study staff except for the pharmacists. No information on how this was done. Drop outs reported: Phase 1: died (n=34), lost to follow up (n=9). Phase 2: died (n=16), lost to follow up (n=31). Power calculations were performed and sufficient sample numbers acquired. Unspecified. Unspecified. No consort flow diagram. 139 eligible subjects randomized. 94 completed the 3 year analysis. 43 dropped out - unrelated deaths (n=28), relocation (n=15). Very high level of deaths despite excluding for co-morbidities. Analysis on 49 (intervention) and 45 (control). In drop-out subjects, there was no statistically significant difference in key demographics. Not clear how many were in each group at baseline as numbers in chart incorrect. After randomization, there was limited separate reporting on the outcome of the six sub-groups. The reasons for these omissions are thought to be that the author was focusing on the placebo or folic acid group (with our without the two different doses of aspirin) as a whole. Recurrent adenomas reported as mean number rather than relative risk. Data from paper cannot therefore be used in meta-analysis. Inconsistencies in baseline data provided. No power calculations were performed. Logan et al. (2008)18 Computergenerated randomized list with a block size of 8, with randomization stratified by centre. The researchers and all clinical staff involved with patient recruitment were blind to this treatment allocation schedule. All trial medication was bottled, labelled and supplied from the central trial pharmacy and dispensed to patients through regional pharmacies at each participating centre. Wu et al. (2009)20 Random number generator No information provided. Aspirin and folic acid placebo were identical in appearance to active treatments. Study participants, clinical staff involved with patient surveillance and investigators at the coordinating centre were all blind to treatment. Only trial pharmacists had knowledge of treatment. No blinding of participants because the control group received no placebo supplement. No further information provided Drop out data provided and largely balanced across two groups. Folate only intervention - 19 drop outs at stage 1 - died (n=4), lost to follow up (n=5), poor health (n=6), patients refused (n=4). 54 drop outs at stage 2 - withdrew from both medication arms (n=42), withdrew from aspirin only (n=11), withdrew from folic acid only (n=1); Placebo Intervention - 29 drop outs at stage 1 - died (n=7), lost to follow up (n=7), poor health (n=7), patient refused (n=8). 46 drop outs at stage 2 - withdrew from both medication arms (n=25), withdrew from aspirin only (n=21). Apparently free of selective outcome reporting - all stated outcomes reported. Power calculations provided but required sample size not met. Drop out figures were provided and reasons for drop out reported. Difference in drop out figures between groups: n=41 discontinued folic acid intervention v n=62 in intervention group. Similar numbers in final analysis cohorts (n=237 in folic acid intervention and n=238 in cohort). Although number of subjects excluded from analysis were similar in both groups it was not explained how these Apparently free of selective outcome reporting - all stated outcomes reported. No inclusion of power calculations. Bonelli et al. (2012)21 No further information provided than randomization lists stratified by centre were provided by the pharmaceutical company. Each centre received a random list where the assigned treatment was indicated by the label numbers of the bottles containing the pills with no reference to their contents. The active compound and the placebo had an identical appearance and that the bottles and pills contained no reference to their contents. Double blinded but no further information Limburg et al. (2011)23 "Dynamic allocation procedure". No information provided. Some interventions were delivered as tablets and some as powder. Also different daily dosages used. related to drop out figures. Baseline characteristics of participants included in the final analysis were similar to all randomly assigned participants. In the 194 participants who did not receive an endoscopy, characteristics such as age or severity of adenoma at baseline, that may have affected the likelihood of a follow up, did not differ significantly at baseline. States that the trial was double blinded but no further information about blinding of personnel was provided. No information provided. All drop outs reported: intervention (n=36): refused follow up colonoscopy (n=29), died (n=6), cancer (n=1); control (n=45): refused follow up colonoscopy (n=35), died (n=9), cancer (n=1). Apparently free of selective outcome reporting - all stated outcomes reported. Low drop-out rate and drop out reasons provided: lost to follow up (n=3), withdrew consent (n=2), medical contraindications (n=1), other (n=2). Apparently free of selective outcome reporting - all stated outcomes reported. Power calculations provided but required sample size not met. No collection of information on confounding factors at baseline. Power calculations provided but required sample size not met. Principi et al. (2013)24 Computer generated. Unspecified. States that the patients and investigators were blinded to assignment without providing any further information. States that both LI and intensity staining were calculated by two independent observers in a blinded fashion without providing any further information. Shimizu et al. (2008)22 Not described. Unspecified. No blinding of participants because the control group received no placebo supplement. States that endoscopists were blinded to the study group but no further information provided. Much higher percentage withdrawal in intervention than control group. 7 out of 30 patients withdrew from the intervention group (4 withdrew consent before T60 colonoscopy and 3 were lost to T60 colonoscopy). 3 out of 30 patients withdrew from the control group (3 withdrew consent before T60 colonoscopy). 8 patients were lost to follow up in intervention arm only as they could not be reached from the day after the initiation of the trial. No intention to treat analysis included. Author refers to it in the discussion and states that the results were insignificant. Apparently free of selective outcome reporting - all stated outcomes reported. Power calculations provided but required sample size not met. Apparently free of selective outcome reporting - all stated outcomes reported. Power calculations not provided.






