Study Design - Duke University Medical Center Library
advertisement

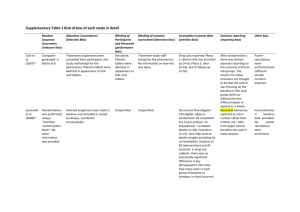
Study Design Meta Analysis A quantitative method of combining the results of independent studies (usually drawn for the published literature) and synthesizing summaries and conclusions which may be used to evaluate therapeutic effectiveness, plan new studies, etc. Meta-analysis aims to produce its conclusion by using statistical methods to combine data from two or more existing studies and reporting the results as if they were from one larger study. Example of meta analysis: Kozyrskyj AL. et al. Treatment of acute otitis media with a shortened course of antibiotics: a meta-analysis. JAMA 279(21):1736-42, 1998 Jun 3. Systematic Review A systematic review usually focuses on a clinical topic and answers a specific question. An extensive review of the evidence is presented after the methodology for identifying the studies in the literature is presented. Example of systematic review: McDonald M. et al. Single- versus multiple-dose antimicrobial prophylaxis for major surgery: a systematic review. Australian & New Zealand Journal of Surgery 68(6):388-96, 1998 Jun. Randomized Controlled Clinical Trial/Controlled Clinical Trial (therapy, diagnosis) A clinical trial involving one or more test treatments, at least one control treatment, specified outcome measures for evaluating the studied intervention, and a bias free method for assigning patients to the test treatment. The treatment may be drugs, devices, or procedures studied for diagnostic, therapeutic or prophylactic effectiveness. Control measures include placebos, active medicine, no treatment, dosage forms and regiments, historical comparisons, etc. When randomization using mathematical techniques, such as the use of a random numbers table, is employed to assign patients to test or control treatments, the trial is characterized as a RANDOMIZED CONTROLLED TRIAL. Trials employing treatment allocation methods such as coin flips, odd-even numbers, patient social security numbers, days of the week, medical record numbers, or other such pseudo- or quasi-random processes are simply designated as CONTROLLED CLINICAL TRIALS. Example of a randomized controlled trial: Macknin ML, Medendorp SV, and Mason P. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Annals of Internal Medicine 125(2):81-8, 1996 Jul 15. Advantages: unbiased distribution of confounders blinding more likely randomization facilitates statistical analysis Disadvantages: expensive: time and money volunteer bias ethically problematic at times Multicenter Studies Controlled studies which are planned and carried out by several cooperating institutions to assess certain variables and outcomes in specific patient populations, for example, a multicenter study of congenital anomalies in children. Crossover Design The administration of two or more experimental therapies one after the other in a specified or random order to the same group of patients. Advantages: all subjects serve as own controls and error variance is reduced thus reducing sample size needed all subjects receive treatment (at least some of the time) statistical tests assuming randomization can be used blinding can be maintained Disadvantages: all subjects receive placebo or alternative treatment at some point washout period lengthy or unknown cannot be used for treatments with permanent effects Cohort Study (prognosis, harm/etiology, prevention) Studies in which subsets of a defined population are identified. These groups may or may not be exposed to factors hypothesized to influence the probability of the occurrence of a particular disease or other outcome. Cohorts are defined populations which, as a whole, are followed in an attempt to determine distinguishing subgroup characteristics. Example of a cohort study: Johansen C and Olsen JH. Risk of cancer among Danish utility workers--a nationwide cohort study. American Journal of Epidemiology 147(6):548-55, 1998 Mar 15. Advantages: ethically safe subjects can be matched can establish timing and directionality of events eligibility criteria and outcome assessments can be standardized administratively easier and cheaper than RCT Disadvantages: controls may be difficult to identify exposure may be linked to a hidden confounder blinding is difficult randomization not present for rare disease, large sample sizes or long follow-up necessary Longitudinal Studies Studies in which variables relating to an individual or group of individuals are assessed over a period of time. Follow-up Studies Studies in which individuals or populations are followed to assess the outcome of exposures, procedures, or effects of a characteristic, e.g., occurrence of disease. Prospective Studies Observation of a population for a sufficient number of persons over a sufficient number of years to generate incidence or mortality rates subsequent to the selection of the study group. Case-control Study (prognosis, harm/etiology, prevention) Studies which start with the identification of persons with a disease of interest and a control (comparison, referent) group without the disease. The relationship of an attribute to the disease is examined by comparing diseased and non-diseased persons with regard to the frequency or levels of the attribute in each group. Involves identifying patients who have the outcome of interest (cases) and control patients without the same outcome, and looking back to see if they had the exposure of interest. Example of a case control study: Del Amo J, Petruckevitch A, Phillips AN, De Cock KM, Stephenson J, Desmond N, Hanscheid T, Low N, Newell A, Obasi A, Paine K, Pym A, Theodore C, and Johnson AM. Risk factors for tuberculosis in patients with AIDS in London: a case-control study. International Journal of Tuberculosis & Lung Disease 3(1):12-7, 1999 Jan. Advantages: quick and cheap only feasible method for very rare disorders or those with long lag between exposure and outcome fewer subjects needed than cross-sectional studies Disadvantages: reliance on recall or records to determine exposure status confounders selection of control groups is difficult potential bias: recall, selection Retrospective Studies Studies used to test etiologic hypotheses in which inferences about an exposure to putative causal factors are derived from data relating to characteristics of persons under study or to events or experiences in their past. The essential feature is that some of the persons under study have the disease or outcome of interest and their characteristics are compared with those of unaffected persons. Cross-Sectional Study Studies in which the presence or absence of disease or other health-related variables are determined in each member of the study population or in a representative sample at one particular time. This contrasts with LONGITUDINAL STUDIES which are followed over a period of time. The observation of a defined population at a single point in time or time interval. Exposure and outcome are determined simultaneously. Example of a cross-sectional study: Abrams K, Skolnik N, and Diamond JJ. Patterns and correlates of tobacco use among suburban Philadelphia 6th through 12th grade students. Family Medicine 31(2):12832, 1999 Feb. Advantages: cheap and simple ethically safe Disadvantages: establishes association at most, not causality recall bias susceptibility confounders may be unequally distributed Neyman bias group sizes may be unequal Review An article published after examination of published material on a subject. It may be comprehensive to various degrees and the time range of material scrutinized may be broad or narrow, but the reviews most often desired are reviews of the current literature. The textual material examined may be equally broad and can encompass, in medicine specifically, clinical material as well as experimental research or case reports. State-of-the-art reviews tend to address more current matters. Reviews of the literature must be differentiated from historical reviews on the same subject, but a review of historical literature is also with the scope of this publication type. Specific headings for specific types of reviews (academic, literature, multicase, reported cases, and tutorial) are also available. Example of a review: Carlsen KH. Exercise induced asthma in children and adolescents and the relationship to sports. [Review] Pediatric Allergy & Immunology 9(4):173-80, 1998 Nov. Study Design Terminology Double-Blind Method -A method of studying a drug or procedure in which both the subjects and investigators are kept unaware of who is actually getting which specific treatment. Match-Pair Analysis -A type of analysis in which subjects in a study group and a comparison group are made comparable with respect to extraneous factors by individually pairing study subjects with the comparison group subjects (e.g., age-matched controls). Meta-Analysis - A quantitative method of combing the results of independent studies (drawn from the published literature) and synthesizing summaries and conclusions which may be used to evaluate therapeutic effectiveness, plan new studies, etc. It is often an overview of clinical trials. It is usually called a meta-analysis by the author or sponsoring body and should be differentiated from reviews of literature. Random Allocation - A process involving chance used in therapeutic trials or other research endeavor for allocating experimental subjects, human or animal, between treatment and control groups, or among treatment groups. It may also apply to experiments on inanimate objects. Reproducibility of Results - The statistical reproducibility of measurements (often in a clinical context), including the testing of instrumentation or techniques to obtain reproducible results. The concept includes reproducibility of physiological measurements, which may be used to develop rules to assess probability or prognosis, or response to a stimulus; reproducibility of occurrence of a condition; and reproducibility of experimental results. Sample Size - The number of units (persons, animals, patients, specified circumstances, etc.) in a population to be studied. The sample size should be big enough to have a high likelihood of detecting a true difference between two groups. Sensitivity and Specificity - Measures for assessing the results of diagnostic and screening tests. Sensitivity represents the proportion of truly diseased persons in a screened population who are identified as being diseased by the test. It is a measure of the probability of correctly diagnosing a condition. Specificity is the proportion of truly nondiseased persons who are so identified by the screening test. It is a measure of the probability of correctly identifying a nondiseased person. Predictive Value of tests - In screening and diagnostic tests, the probability that a person with a positive test is a true positive (i.e., has the disease), is referred to as the predictive value of a positive test; whereas, the predictive value of a negative test is the probability that the person with a negative test does not have the disease. Predictive value is related to the sensitivity and specificity of the test. ROC Curve - A graphic means for assessing the ability of a screening test to discriminate between healthy and diseased persons; may also be used in other studies, e.g., distinguishing stimuli responses as to a faint stimuli or nonstimuli. Single-Blind Method - A method in which either the observer(s) or the subject(s) is kept ignorant of the group to which the subjects are assigned. [This information is taken from the Evidence-Based Medicine Glossary at Oxford University and the MeSH scope notes from the National Library of Medicine.]