Periodic Behavior of Metals
advertisement
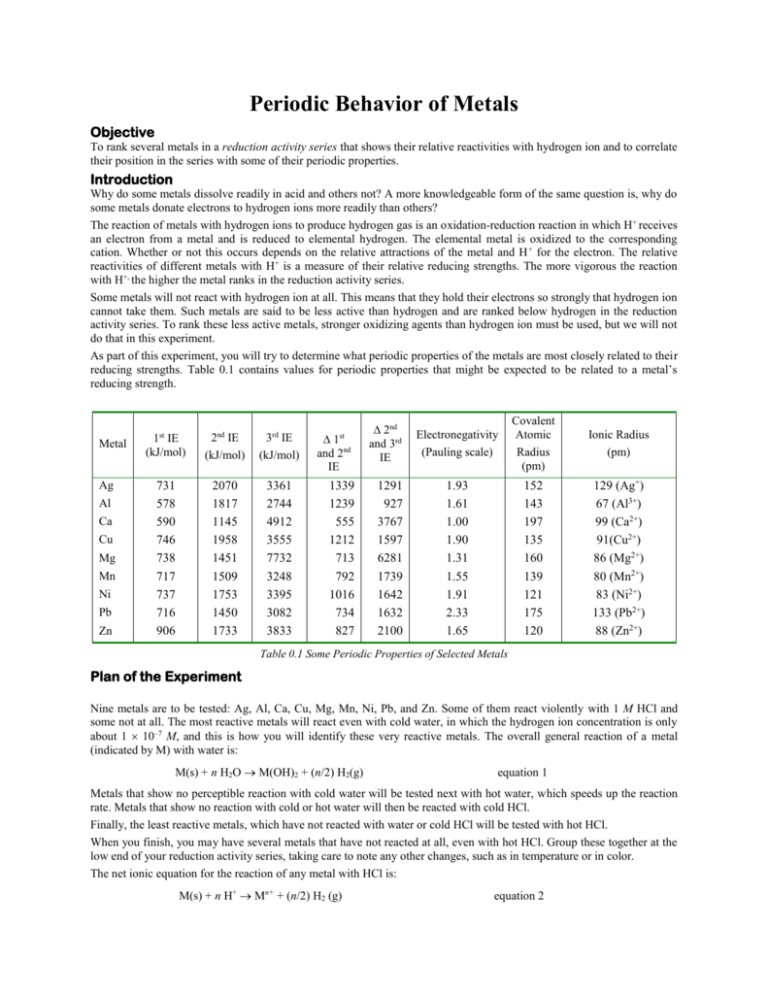
Periodic Behavior of Metals Objective To rank several metals in a reduction activity series that shows their relative reactivities with hydrogen ion and to correlate their position in the series with some of their periodic properties. Introduction Why do some metals dissolve readily in acid and others not? A more knowledgeable form of the same question is, why do some metals donate electrons to hydrogen ions more readily than others? The reaction of metals with hydrogen ions to produce hydrogen gas is an oxidation-reduction reaction in which H+ receives an electron from a metal and is reduced to elemental hydrogen. The elemental metal is oxidized to the corresponding cation. Whether or not this occurs depends on the relative attractions of the metal and H + for the electron. The relative reactivities of different metals with H+ is a measure of their relative reducing strengths. The more vigorous the reaction with H+, the higher the metal ranks in the reduction activity series. Some metals will not react with hydrogen ion at all. This means that they hold their electrons so strongly that hydrogen ion cannot take them. Such metals are said to be less active than hydrogen and are ranked below hydrogen in the reduction activity series. To rank these less active metals, stronger oxidizing agents than hydrogen ion must be used, but we will not do that in this experiment. As part of this experiment, you will try to determine what periodic properties of the metals are most closely related to their reducing strengths. Table 0.1 contains values for periodic properties that might be expected to be related to a metal’s reducing strength. Metal Ag Al Ca Cu Mg Mn Ni Pb Zn st nd rd 1 IE (kJ/mol) 2 IE (kJ/mol) 3 IE (kJ/mol) 731 578 590 746 738 717 737 716 906 2070 1817 1145 1958 1451 1509 1753 1450 1733 3361 2744 4912 3555 7732 3248 3395 3082 3833 and 2nd IE st 1339 1239 555 1212 713 792 1016 734 827 2nd and 3rd IE 1291 927 3767 1597 6281 1739 1642 1632 2100 Electronegativity (Pauling scale) Covalent Atomic Radius (pm) Ionic Radius (pm) 1.93 1.61 1.00 1.90 1.31 1.55 1.91 2.33 1.65 152 143 197 135 160 139 121 175 120 129 (Ag+) 67 (Al3+) 99 (Ca2+) 91(Cu2+) 86 (Mg2+) 80 (Mn2+) 83 (Ni2+) 133 (Pb2+) 88 (Zn2+) Table 0.1 Some Periodic Properties of Selected Metals Plan of the Experiment Nine metals are to be tested: Ag, Al, Ca, Cu, Mg, Mn, Ni, Pb, and Zn. Some of them react violently with 1 M HCl and some not at all. The most reactive metals will react even with cold water, in which the hydrogen ion concentration is only about 1 10–7 M, and this is how you will identify these very reactive metals. The overall general reaction of a metal (indicated by M) with water is: M(s) + n H2O M(OH)2 + (n/2) H2(g) equation 1 Metals that show no perceptible reaction with cold water will be tested next with hot water, which speeds up the reaction rate. Metals that show no reaction with cold or hot water will then be reacted with cold HCl. Finally, the least reactive metals, which have not reacted with water or cold HCl will be tested with hot HCl. When you finish, you may have several metals that have not reacted at all, even with hot HCl. Group these together at the low end of your reduction activity series, taking care to note any other changes, such as in temperature or in color. The net ionic equation for the reaction of any metal with HCl is: M(s) + n H+ Mn+ + (n/2) H2 (g) equation 2 Caution! Combining highly reactive metals with hydrochloric acid is dangerous. Hydrochloric acid may cause chemical burns. Calcium metal may also casue chemical burns. Procedure 1.) Fill a 400 mL beaker 2/3 full of water and heat it to near boiling. It will be used as a water bath for various samples throughout the experiment. Try to maintain the temperature just below the boiling point. Refill the bath during the experiment when too much water has boiled away. 2.) Obtain powdered or granular samples of the following metals: Al, Ca, Cu, Mg, Mn, Ni, Pb, Zn 3.) Test each metal, except aluminum, by putting a small amount of each in large labeled test tubes containing about 5 mL of cold distilled water. Observe whether any bubbles of H2 form. Record which metals react, ranking them in order of most vigorous reactivity. 4.) Aluminum requires special treatment to remove its thin film of oxide before it can be tested. a.) Put about a centimeter of Al in a large test tube with 2 mL of H2O and 2 mL of 6 M HCl. b.) Heat the test tube and contents in the water bath for a few minutes until a vigorous reaction is observed. Immediately remove the test tube and quickly add about 10 mL of cold distilled water to dilute the acid solution, then decant the diluted acid into a waste beaker. Caution! Do not allow the acid/aluminum mixture to boil. Doing so will may cause the mixture to erupt, and this may burn your skin. It will certainly make quite a mess to clean up. 5.) 6.) 7.) 8.) 9.) 10.) c.) Wash the Al granules twice as follows: fill the test tube half full with distilled water, decant most of the water into the waste beaker leaving enough water to completely cover the metal pieces. Repeat the washing. d.) Observe whether Al reacts with the cold water remaining in the test tube. Record your observations and rank aluminum in the cold water reactivity series. For only those metals that do not react or react very weakly with cold water, place their test tubes in the hot water bath. Observe whether bubbles form and record the results, again ranking the metals by the vigor of reaction. Using only the metals that have not yet reacted, pour off all but about 2 mL of water and add 2 mL of 6 M HCl. Watch for three minutes, if necessary, for signs of reactivity. Record the relative bubbling rates. For any metals that did not react with cold HCl, place their test tubes containing cold HCl in the hot water bath (not quite boiling) and watch for any reaction. Record the relative bubbling rates. In your Lab Notebook, arrange the metals in order of reduction activity, putting the most reactive first. Group all completely non-reactive metals together at the end of the list. Your TA will test silver (it’s too expensive for everyone to test!) and will report the results to the class. Using Table 1, try to find the periodic property that correlates best with your reduction activity series. Plot the value of the periodic property on the vertical axis and the ordered list of metals on the horizontal axis. Do not look for an exact correlation because many other factors may influence reactivity. Look for the periodic property that shows the best correlation. Use your knowledge of chemistry to provide an explanation for the correlation you observe. Why does one property fit better than the others?







