Force Field Exercise
advertisement
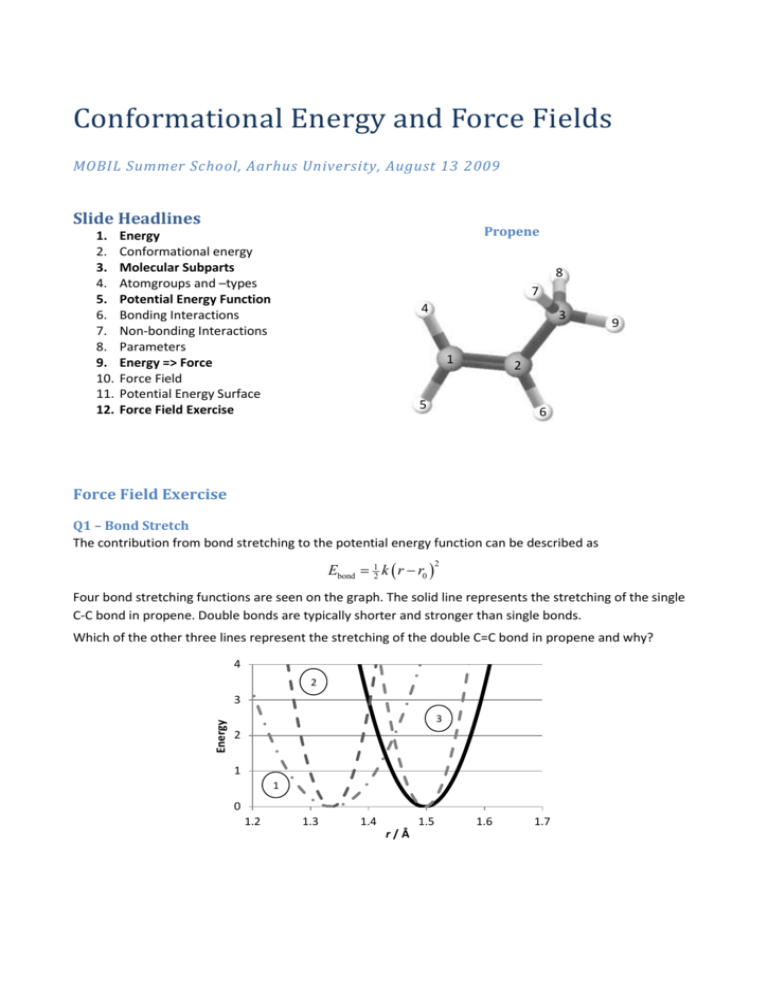
Conformational Energy and Force Fields MOBIL Summer School, Aarhus University, August 13 2009 Slide Headlines 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Propene Energy Conformational energy Molecular Subparts Atomgroups and –types Potential Energy Function Bonding Interactions Non-bonding Interactions Parameters Energy => Force Force Field Potential Energy Surface Force Field Exercise 8 7 4 3 1 9 2 5 6 Force Field Exercise Q1 – Bond Stretch The contribution from bond stretching to the potential energy function can be described as Ebond 12 k r r0 2 Four bond stretching functions are seen on the graph. The solid line represents the stretching of the single C-C bond in propene. Double bonds are typically shorter and stronger than single bonds. Which of the other three lines represent the stretching of the double C=C bond in propene and why? 4 2 Energy 3 3 2 1 1 0 1.2 1.3 1.4 1.5 r/Å 1.6 1.7 Q2 – Bond Rotation The contribution from bond rotation to the potential energy function can be described as Ebondrot 12 Vn 1 cos n n where n is the number of energy minima encountered during a 360° rotation around the bond. Two bond rotation functions are seen on the graph. One of them represents the H6-C2-C3-H9 contribution to the rotation around the single bond in propene, the other represents the H5-C1-C2-H6 contribution to the rotation around the double bond. Which line represents the single bond, which represents the double bond and why? 12 2 Energy 8 4 1 0 0 60 120 180 240 ω/° 300 360 Advanced follow up: Here was shown only one contribution to the potential energy profile for rotating around the bonds. How many interactions contribute in fact to the rotation around the single and the double bonds? Q3 – VdW Interactions The contribution from Van der Waals interactions to the potential energy function can be described as EvdW 6 Rmin 12 Rmin 2 r r The graphs represent the vdW interaction between two hydrogens and between a hydrogen and a carbon (sp3). Which line represents the H-H interaction, which represents the C-H interaction and why? 0.25 0.2 Energy 0.15 2 0.1 0.05 0 -0.05 1 2 3 4 5 r/Å Q4 – Force Field What constitutes a force field, and why does it make sense to call it a ”force field”?






