Radioactive Decay Lab
advertisement

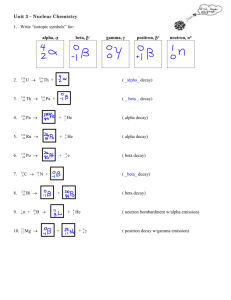
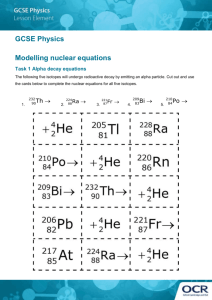
5.1.2 Lab: Nuclear Decay Chain Points possible: 40 Lab Name: ____________________ Date: ___________ Period: ________ Materials: The periodic table of elements. Investigation: 1. Make your prediction in the space provided below. 2. Begin filling in the decay chain of uranium-238 to lead-206. Make sure that you pay attention to the type of decay at each step (alpha or beta), and use the periodic table to find the names of the elements at each step. 3. Check that the number of protons and neutrons in the nucleus always match the nucleon number of the element. The decay series should transition smoothly all the way to lead-206, so make sure the final step does, in fact, end with the correct number of protons and neutrons to make lead-206. 4. Answer the set of summary questions on your lab report, and turn in the completed lab to your instructor. Question: How many different elements appear in the Uranium-238 decay chain series? Use your knowledge of alpha and beta decay to make an educated guess. Explain your prediction. (3 points) Prediction: Data: Fill in the chart on the following pages. For each of the fourteen steps in the decay series, there is either an alpha or beta particle emitted. (26 points) Alpha decay: Two protons and two neutrons are emitted as the alpha particle. This makes the total nucleon number drop by four. Beta decay: One neutron turns into an electron and a proton. The electron is emitted as the beta particle, while the neutron is exchanged for a proton in the nucleus. The nucleon number stays the same. You need to provide the element name followed by its nucleon number, for example, "Uranium-238." Also write the number of protons and neutrons in the nucleus at each stage. The first and last stages of the decay series have been filled in as examples for you to follow. Summary 1. How many different elements appear in the decay chain? Does this match your original prediction? List the elements and explain your answer. (2 points) 2. Which element occurs the most number of times? Of the elements that appear more than once, do any of them appear more than once as the exact same isotope? (i.e. same number of protons and neutrons)? (2 points) 3. Does there seem to be any pattern in the series of alpha and beta decays? (2 points) 4. Does the total number of nucleons in the nucleus of the atom ever increase during the decay chain? Do you think it could happen with any kind of decay process? (Hint: Use your knowledge of conservation of mass.) (2 points) 5. Do you think the decay chain could continue on further? Why do you think it stops at lead-206? List the additional two elements and nucleon numbers in the decay chain if there were two more alpha decay steps after lead-206. (2 points) 6. What is another name for the alpha particle that is emitted during alpha decay? (1 point)