bit25112-sm-0001-SupFig-S1
advertisement

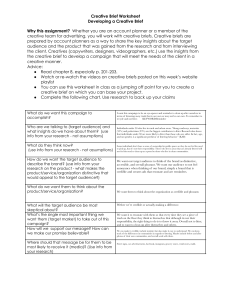
Supplementary Material Improved Isoelectric Focusing Chromatography on Strong Anion Exchange Media via a New Model that Custom Designs Mobile Phases using Simple Buffers Derek Y.C. Choy, A. Louise Creagh, and Charles Haynes* Michael Smith Laboratories, and the Department of Chemical and Biological Engineering, University of British Columbia, Vancouver, BC V6T 1Z4, Canada * Address all correspondence to: Professor Charles Haynes Michael Smith Laboratories 231 Michael Smith Building University of British Columbia Vancouver, BC V6T 1Z4 Canada Email: israels@chbe.ubc.ca S1. Additional Materials and Methods Used Materials ReadyGel isoelectric focusing (IEF) gels and pH 5-8 buffer, 10x cathode buffer, 10x anode buffer, IEF sample buffer, buffer standards from pH 4.45 to 9.6, and Coomassie Brilliant Blue R-250 were purchased from BioRad Inc. Crocein scarlet was purchased from Sigma. Methanol and 2-propanol were obtained from Fisher. The IEF staining solution consisted of 27% (v/v) 2-propanol, 10% (v/v) acetic acid, 0.04% (v/v) Coomassie Blue R-250 and 0.05% (v/v) crocein scarlet in nanopure water, while the destaining solution consisted of 40% (v/v) methanol and 10% (v/v) acetic acid in nanopure water. All buffer solutions used as mobile phases for column equilibration and sample loading/ elution were prepared with nanopure water, adjusted to the desired pH with concentrated HCl, and then filtered through a 0.22 m Durapore® PVDF membrane (Millipore) and vacuum degassed. Diethanolamine, 1,3-diaminopropane, bis-tris, trizma hydrochloride, imidazole, piperazine and lactic acid were obtained from Sigma. Glacial acetic acid, hydrochloric acid and potassium phosphate monobasic were obtained from Fisher. Myoglobin, carbonic anhydrase, conalbumin, -lactalbumin, bovine serum albumin, ovalbumin, -amylase, trypsin inhibitor, and -lactoglobulin B were obtained from Sigma. Glucose oxidase was obtained from Fluka. Gel electrophoresis procedures A Mini-Protean III electrophoresis unit from BioRad was used to perform gel isoelectric focusing (IEF) of each model protein. Protein samples were prepared in IEF sample buffer to final concentrations of 10 mg mL-1 protein and 5% (w/v) glycerol. Each gel lane was loaded with either 10 L of protein sample or 3 L of IEF standard mixture. Electrophoresis was performed at 100 V for 60 minutes, and then at 250 V for 60 minutes using a Power Station 300 power supply from Labnet International Inc. A final migration at 500 V for 30 minutes using a FB500 power supply from Fisher Biotech was then conducted. The focused gel was stained with IEF staining solution for 30 minutes and then destained overnight. Mass spectrometry Aliquots (0.2 L) of protein fractions collected from chromatofocusing eluents were lyophilized using a SC110A vacuum concentrator and UVS400 vacuum system from Thermo Electron Corp., and then resuspended at appropriate concentration in nanopure water. Intact protein mass analysis was performed on a Voyager-DE STR MALDI-TOF (Applied Biosystems). Tryptic-digested proteins were analyzed for sequence on an API Q STAR PULSARi Hybrid LC/MS/MS from LC Packings and Applied Biosystems. A MASCOT database search was performed for protein identification. Figure 7: Isoelectric focusing (IEF) gel for 10 μL protein (10 mg mL-1) samples of myoglobin, carbonic anhydrase, conalbumin, bovine serum albumin, ovalbumin, β-amylase, trypsin inhibitor, β-lactoglobulin A, β-lactoglobulin B and α-lactalbumin (lanes 2 to 11). Samples were loaded onto a ReadyGel IEF gel pH 5-8 and electrophoresed in a Mini-Protean III unit. IEF protein standards (3 µL) containing cytochrome C (pI = 9.6), lentil lectin (pI’s = 7.80, 8.00 and 8.20), human hemoglobulin C (pI = 7.5), human hemoglobulin A (pI = 7.1), equine myoglobin (pI’s = 6.8 and 7.0), human carbonic anhydrase (pI = 6.5), bovine carbonic anhydrase (pI = 6.0), βlactoglobulin B (pI = 5.1) and phycocyanin (pI’s = 4.45, 4.65 and 4.75) were loaded to lanes 1 and 12 for reference.