Metabolic Signatures of Adiposity and Weight Change in Young

Metabolic Signatures of Adiposity and Weight Change in Young Adults:
Observational and Genetic Evidence
Peter Würtz*, PhD, Qin Wang, MSc, Antti J. Kangas, MSc, Joni Skarp, BM, Mika Tiainen, PhD, Tuulia
Tynkkynen, PhD, Pasi Soininen, PhD, Aki S. Havulinna, PhD, Marika Kaakkinen, PhD, Rebecca C.
Richmond, MSc, Jorma S. Viikari, MD PhD, Markku Savolainen, MD PhD, Mika Kähönen, MD PhD,
Terho Lehtimäki, MD PhD, Jaana Laitinen, MD PhD, Anneli Pouta, MD PhD, Pekka Mäntyselkä, MD
PhD, Paul Elliott, MB, PhD, Kirsi Pietiläinen, MD PhD, Mauno Vanhala, MD PhD, George Davey
Smith, MD PhD, Samuli Ripatti, PhD, Veikko Salomaa, MD PhD, Olli T. Raitakari, MD PhD, Marjo-
Riitta Järvelin, MD PhD, Mika Ala-Korpela, PhD
1.
Institute for Molecular Medicine Finland, University of Helsinki, Finland
2.
Computational Medicine, Institute of Health Sciences, University of Oulu, Oulu, Finland
3.
NMR Metabolomics Laboratory, School of Pharmacy, University of Eastern Finland, Kuopio,
Finland
4.
Department of Chronic Disease Prevention, National Institute for Health and Welfare, Helsinki,
Finland
5.
Medical Research Council Centre for Causal Analyses in Translational Epidemiology, University of Bristol, Bristol, United Kingdom
6.
Department of Medicine, University of Turku and Turku University Hospital, Turku, Finland
7.
Department of Clinical Physiology, University of Tampere and Tampere University Hospital,
Tampere, Finland
8.
Department of Clinical Chemistry, University of Tampere and Tampere University Hospital,
Tampere, Finland
9.
Finnish Institute of Occupational Health, Helsinki, Finland
10.
Department of Children, Young People and Families, National Institute for Health and
Welfare, Finland
11.
Institute of Clinical Medicine, University of Oulu, Oulu, Finland
12.
Institute of Clinical Medicine, University of Turku, Finland; Primary Health Care Unit, Turku
University Hospital, Turku, Finland
13.
School of Medicine, University of Eastern Finland, Kuopio, Finland
14.
Obesity Research Unit, Department of Medicine, Helsinki University Central Hospital
15.
Wellcome Trust Sanger Institute, Hinxton, United Kingdom
16.
Department of Epidemiology and Biostatistics, Imperial College London, United Kingdom
17.
Centre for Environment and Health, Imperial College London, London, United Kingdom
18.
Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku,
Turku, Finland
19.
Department of Clinical Physiology, Turku University Hospital, Turku, Finland
20.
Department of Life Course and Services, National Institute for Health and Welfare, Oulu,
Finland
21.
Institute of Health Sciences and Biocenter, University of Oulu, Oulu, Finland
1
Running title: Metabolic Signatures of Adiposity
Word count (excluding Abstract, Tables, Figure Captions, and References): XXXX words; Word count including all: X Tables, X Figures, Supplementary Material: X Tables, X Figures.
*Corresponding author:
Peter Würtz, PhD, Institute for Molecular Medicine Finland, PO Box 20, Tukholmankatu 8, 00014
Univ Helsinki, Finland; peter.wurtz@helsinki.fi; Phone: +358 5046 70900; Fax: +358 2061 08480
Reprint requests: Peter Würtz; Disclosures: None
Todo
Sensitivity: GPS analyses without FTO and with genescore unweighted (b=0.81±0.04, R2=0.83).
Better Justification of the metabolic biomarkers used in the study
Best-fit accounting for correlation structure (general linear model; do fit with SHBG and Testo point separately for men and women)
What’s up with vitamin D? Opposite genetic effect to what was just published in PLoS Med.
Key updates/finalizations
Should we exclude ~1,000 persons with genotypes missing from cross-sectional analyses so that the persons are the same (results similar).
Omit metabolite transformation for simplicity + provide interpretable quantified associations
(results highly similar).
Lipid metabolites and manual genotyped gene-score in NFBC86 => (1000+ more persons).
2
ABSTRACT (350w)
Importance Adiposity is linked with adverse metabolic effects and increased risk for cardiometabolic diseases. Knowledge on the causal relation of elevated body mass index (BMI) on the comprehensive metabolite profile and metabolic effects of weight change could inform prevention policies.
Objective To characterize metabolic signatures of elevated BMI in healthy adolescents and young adults, and compare with the influences of genetic predisposition and weight change.
Design, Setting, and Participants Observational analyses of 14,482 adolescents and young adults (mean age 26±8, range 16–39; 51% women) from four population-based studies in Finland. High-throughput nuclear magnetic resonance spectroscopy and biochemical assays were used to quantify 79 circulating metabolite measures. A genetic-predisposition score to elevated BMI was calculated based of 32 BMIassociated loci. Weight change associations with changes in the metabolome were examined for 1,488 persons during 6-year follow-up.
Main Outcome Measure Quantification of causal effects of adiposity on the systemic metabolite profile.
Results The comprehensive metabolite profile was prominently associated with elevated BMI in the young study population with lipoprotein size and subclass distributions, saturation of fatty acids and amino acids displaying association magnitudes comparable to established metabolic risk factors. Effects of one-unit increment in the genetic-predisposition score, corresponding to one kg/m 2 higher observed BMI, were concordant with causal metabolic effects of elevated BMI and on average 83±4% of the magnitude expected from observational associations (R 2 =0.87). In contrast, the 6-year change in BMI was accompanied by 150±4% changes in metabolite concentrations compared to cross-sectional estimates
(R 2 =0.92). A modest weight loss of 3-6% was accompanied by favorable metabolite changes throughout the systemic metabolite profile, including novel biomarkers for cardiometabolic diseases.
Conclusions and Relevance Increased adiposity within the non-obese weight range exhibited a prominent impact throughout the comprehensive metabolite profile in young adults. Mendelian randomization analyses demonstrate a causal role of elevated BMI on the global cardiometabolic profile and suggest a genetic basis for the risk factors clustering related to obesity. Despite the genetic influences, both weight gain and weight loss were paralleled by greater metabolite changes than anticipated, suggesting a broadly modifiable metabolite profile through weight change.
These findings inform the role of heredity and lifestyle on the metabolic effects of obesity.
3
I NTRODUCTION
The prevalence of obesity is reaching epidemic proportions and represents a major threat to public health worldwide.
National, regional, and global trends/Lancet 2011 Excess bodyweight, as assessed by body mass index (BMI), increases the risk of adverse outcomes including cardiovascular disease, type 2 diabetes, certain cancers and premature death.
Prospective Studies Collaboration 2009, Lewis Circ 2009, deGonzales NEJM
The increased morbidity and mortality conferred by elevated BMI is partly ascribed to effects on lipid and glucose levels as well as hypertension.
Prospective Studies Collaboration 2009 However, obesity is not only associated with traditional cardiovascular risk factors, but has been linked with global perturbations in the metabolite profile.
Newgard2009, Pietiläinen2011 Detailed studies on metabolic signatures of obesity have previously focused primarily on small population groups of extreme body composition.
Newgard 2009, Pietiläinen 2011, Mihalik 2012, Laferrère 2011, Wahl“KORA”2012 Yet, it unclear whether such comprehensive metabolic signatures of excess bodyweight are observed within the nonobese range of BMI. Further, it remains uncertain whether the metabolic aberrations related to obesity are caused by increased adiposity or a consequence hereof.
Addressing causal metabolic effects of obesity is challenging in observational studies, due to potential confounders, bias and reverse causation. Randomized trials of weight reduction in obese individuals indicate favorable effects on conventional risk factors, Tuomilehto, Wood, Williams1990, Knowler however these have predominantly been conducted among participants in the obese range
(BMI≥30 kg/m 2 ).
Moyer Annals 2012 The wider effects on the systemic metabolite profile have not been investigated in general population settings. Here, we use genetic information on predisposition to elevated BMI to ascertain the causal influence of adiposity on the comprehensive metabolite profile utilizing the framework of Mendelian randomization as an analogue of the randomized trial.
Davey Smith and Ebrahim 2003 Genetic variation in the fat-mass- and obesity-related (FTO) gene has previously been associated with dyslipidemia, hypertension and insulin resistance, Freathy 2008, Timpson
Hypertension 2009 and genetic correlates of adiposity have been linked with increased risk for ischemic heart disease.
Nordestgaard2012, Cardiogram If elevated BMI exerts causal adverse effects on the comprehensive metabolite profile already in early adulthood this highlights weight maintenance and reduction as a key target for effective global risk factor management/control.
Despite the prominent genetic contribution to adiposity, BMI is classified as a major modifiable risk factor. However, the responsiveness of the metabolome to spontaneous weight change has
4
not been investigated in general population settings. Since change in bodyweight in adulthood is primarily environmentally dependent, (is this even true? ref?) comparison of the metabolite changes observed along weight change may elucidate influences of genetic variation and environmentallyinduced changes in BMI on the comprehensive metabolic profile. In order to characterize the metabolic impact of adiposity on the metabolite profile we conducted high-throughput metabolomics of 15,283 adolescents and young adults from four population-based studies in
Finland. The objectives were to 1) to quantify associations of BMI with systemic metabolite levels,
2) to examine the effects of genetic predisposition to elevated BMI on the metabolite concentrations, and 3) to assess the responsiveness of the metabolite profile upon weight change during 6-year follow-up. The genetic and longitudinal estimates were compared to cross-sectional observations to quantify the causal metabolic effects of adiposity and clarify the holistic metabolic consequences of weight loss in young adults.
METHODS
Study populations. The study comprised four general population cohorts from Finland: the
Northern Finland Birth Cohorts of 1986 (n=5,225 adolescents aged 16) Kantomaa2012 and 1966
(n=5,546 persons 31 years of age) Sabatti NG2009 , the Cardiovascular Risk in Young Finns Study (YFS, n=2,367 individuals aged 24-39) Raitakari 2008 , and FINRISK 1997 (n=2,145 persons aged 24-
39).
Vartiainen2010 To minimize the confounding effects of aging, as well as to limit dilution of genetic effects, Tikkanen2011 individuals over the age of 39 were omitted from primary analyses. Pregnant women (n=224) as well as persons with type 1 or type 2 diabetes (n=224) or on lipid or antihypertensive treatment (n=185) were excluded. In total, 15,283 adolescents and young adults with
BMI and systemic metabolite profile measured were included in the present study. A subset of
1,488 persons from YFS attended 6-year follow-up with BMI and metabolite profile quantified.
BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference, blood pressure and various standard biochemical assays were conducted as part of the clinical examination. Smoking status, daily usage of alcohol and physical activity index in terms of metabolic equivalent of task were assessed by questionnaires.
Würtz 2012 An allelic score for genetic-predisposition to elevated BMI was derived for 12,691 participants based on 32 single nucleotide polymorphisms previously correlated with BMI in large-scale genome-wide association studies.
Speliotes.NG2010, Qi2012 Details of the study cohorts, biochemical assays, and genotyping are
5
described in the Supplementary Online Content. All participants gave written informed consent, the study protocols were approved by the local ethics committees.
Metabolite quantification. A high-throughput nuclear magnetic resonance (NMR) spectrometer operating at 500 MHz was used for quantification of circulating metabolites.
Soininen 2009, Inouye 2010
Three NMR spectra were recorded from each serum sample for measurement of lipoprotein subclass distributions, fatty acid composition and degree of saturation, as well as various small molecules including amino acids and glycolysis precursors ( eTable 1 ). Metabolite quantification in mmol/L was conducted by regression modeling. The majority of serum samples were drawn after overnight fast (85%). The metabolite profiling platform has previously been employed in various epidemiological and genetic studies (Würtz 2012, Kettunen 2012) and details of the experimentation have been described (Soininen 2009, Inouye 2010).
Statistical analyses. Metabolites were cubic root-transformed prior to analyses. All metabolite measures were standardized to standard deviation (SD) levels separately for each cohort. The corresponding absolute concentrations are shown in eTable 1. For each metabolite, a multiple linear regression model was fitted with BMI as the explanatory variable and the metabolite concentration as outcome. The associations were adjusted for sex, age (where appropriate), physical activity index, current smoking status and alcohol intake. Results were analyzed separately for the four cohorts and combined using inverse-variance weighted meta-analysis assuming random effects. Associations were regarded significant if the meta-analyzed P-value was below 0.0005 to account for 84 independent tests. The cross-sectional metabolite associations with BMI were examined for consistency across the four cohorts and further for generalizability in two older populations (Supplementary Methods).
The combined effect of 32 genetic variants previously correlated with BMI was used to derive a genetic-predisposition score for elevated BMI. The score was calculated as the number of
BMI-correlated alleles weighted by the effect size on BMI previously determined in genome-wide association meta-analysis.
Speliotes.NG2010
The association of the genetic-predisposition score with observed BMI was assessed, and the score was subsequently scaled so that 1-unit [kg/m 2 ] increment in the genetic-predisposition score corresponds to 1 kg/m 2 -unit increase in observed
BMI in each cohort. Associations of the genetic-predisposition score with each metabolite were
6
assessed in linear regression models adjusted for the same covariates as in cross-sectional analyses. Associations were meta-analyzed for the four cohorts using random effect inversevariance weighting (n= 12,691 ). The association magnitudes of the genetic-predisposition score with the metabolites were then compared to the corresponding cross-sectional associations.
Utilizing this Mendelian randomization framework (Davey Smith and Ebrahim 2003) , the geneticpredisposition score served as an instrumental variable to assess causality for the effect of elevated BMI on the systemic metabolite profile (eFigure 1) . In the case of a causal role of
adiposity, 1-unit increment in the genetic-predisposition score would be associated with differences in the metabolite concentrations to a similar extent as for the cross-sectional associations of 1-unit increment in observed BMI.
Davey Smith Ebrahim 2003, Freathy 2008, Sheehan PLoS Med 2008 The overall correspondence between the genetic and cross-sectional effects was quantified by the slope of a linear model fitted through the observations of genetic vs. cross-sectional associations.
To further examine whether the genetic effects on the metabolite profile would be mediated through BMI, we tested the genetic associations with observed BMI included in the models and the pair-wise differences between the genetic and cross-sectional associations using a
Z-statistic.
Freathy2008
Data on longitudinal changes in BMI and metabolite levels during 6-year follow-up were tested for a subset of 1,488 individuals from YFS. Longitudinal associations were assessed for each metabolite with a linear regression model using 6-year change in BMI as predictor and 6-year change in metabolite concentration as outcome with adjustment for sex, age, baseline metabolite concentration, change in smoking status, change in physical activity and change in alcohol intake.
To quantify the effect of weight change on the metabolite profile, the standardized metabolite change per to 1 kg/m 2 -unit change in BMI were compared to the corresponding cross-sectional associations of 1-unit increment in BMI. The correspondence between the longitudinal and crosssectional associations was quantified by the slope of a linear model fitted through the longitudinal
vs. cross-sectional observations. For each metabolite, we also tested the difference between the genetic and cross-sectional associations using a Z-statistic. To examine the generalizability, the longitudinal associations were further examined for consistent over 10-year follow-up time in the same study population as well as tested in a independent cohort of 456 middle-aged persons with
6.5-year follow-up.
WürtzDCare2012 Finally, to illustrate the metabolite profile changes paralleled by weight change, the mean changes in metabolite levels were calculated for groups of 3-6% weight
7
loss, 3-6% weight gain and 6-12% weight gain during 6-year follow-up, after adjustment for sex, age, smoking status, physical activity and alcohol intake at both baseline and follow-up.
RESULTS
The study comprised 15,283 adolescents and young adults free of medication for cardiometabolic risk factors. Clinical characteristics of the four population-based cohorts are shown in Table 1. The mean BMI of 25 kg/m 2 observed in the young adults reflects the population average in
Finland.
Peltonen2007 Less than 10% of the study population had BMI>30 kg/m 2 or were classified as having the metabolic syndrome. Mean concentrations of all assayed metabolites and metabolic risk factors are listed in eTable 1. Intercorrelations of the metabolite measures are shown in eFigure 2.
Associations of body mass index with systemic metabolite profile
To characterize metabolite profiles of adiposity we examined associations of BMI with 82 metabolic measures. The majority of the metabolites were quantified by high-throughput profiling
Soininen ; additionally, 14 metabolic measures collected in at least two of the population studies were included as part of the comprehensive metabolite profile. The cross-sectional, genetic, and longitudinal change associations of BMI with the metabolite measures are illustrated in Figure 1.
The cross-sectional associations are shown as white rounded rectangles with the length of the rectangles indicate 95% confidence intervals (CI). The small CIs reflect the generally consistent association estimates across the study populations, including the cohort of 16-year old adolescents (eFigure 3). The majority of metabolites were associated with elevated BMI, with 90% of cross-sectional associations displaying P<0.05 in meta-analysis. Association magnitudes are quantified in standardized units of 1-SD metabolite concentration per increment in observed BMI.
For instance, the cross-sectional association of BMI with plasma insulin indicating 0.12 SD higher insulin per BMI-unit, corresponding to 0.5 IU/L higher insulin per 1-kg/m 2 increment in BMI.
For lipoproteins, most pronounced cross-sectional associations with BMI were observed for very-low-density lipoprotein (VLDL) lipids, whereas associations with low-density lipoprotein (LDL) lipids were weaker. High-density lipoprotein (HDL) lipids displayed a more heterogeneous association pattern with strong inverse associations for large HDL and HDL particle size. Prominent direct associations with BMI were also observed for mono-unsaturated and saturated fatty acids, while e.g. polyunsaturated fatty acids, relative to total fatty acids, were inversely associated with
8
elevated BMI. Weaker associations were observed for metabolites related to glycolysis and gluconeogenesis in the young adults analyzed in this study. In contrast, branched-chain and aromatic amino acids exhibited strong associations with BMI, with association magnitudes comparable to those of cholesterol and triglycerides. In addition, strong associations with BMI were observed for metabolic risk factors such as inflammatory and liver function markers, adipokines and sex hormones. The observed metabolite profile associated with elevated BMI was similar for 4,771 middle-aged and older persons eFigure 2).
Results were essentially similar when replacing BMI for waist circumference or when individuals with BMI>=30 (n=1,049) were omitted from the analyses (data not shown).
Metabolic effects of genetic-predisposition to elevated BMI
The genetic-predisposition score was robustly correlated with BMI (r=0.14; P=4×10 -59 ) and explained 1.3-2.6% of the variation in BMI in the young study population (Table 1). Each increment in the genetic-predisposition score was associated with approximately 1 kg/m 2 unit higher observed BMI; the score was subsequently scaled so this association was exactly 1 unit in each cohort. The genetic-predisposition score was not associated with potential confounders such as smoking and drinking status, socioeconomic status, or physical activity index (eTable 2).
Associations of the genetic-predisposition score for elevated BMI with the systemic metabolite profile are shown as red half circles in Figure 2. The majority of metabolites were associated with the gene score at P<0.05, numerous at the Bonferroni corrected threshold of P<0.0005. The strongest positive associations of the genetic-predisposition score were observed for large VLDL lipid levels, branched-chain and aromatic amino acids as well as well as inflammatory markers, liver enzymes, leptin, and insulin levels. The strongest inverse associations were found for large
HDL and HDL particle size, the ratio of polyunsaturated fatty acids (PUFA) to total fatty acids, and adiponectin. Overall, the associations of the genetic-predisposition score followed an association pattern similar as the cross-sectional observations, albeit the confidence intervals of the genetic effects were wider: the metabolic alterations associated with 1-unit increment in the gene score
(red dots) were analogous to those observed for 1-unit higher cross-sectional BMI (white circles).
Utilizing the Mendelian randomization framework to address causality of the metabolic effects of adiposity, equal association magnitudes between the genetic and cross-sectional observations would suggest that genetic effects on the metabolite profile are mediated through BMI
9
(eFigure 1). To quantify this relation, the cross-sectional observations were compared with the genetic effects as shown in Figure 2A. Testing for differences for each metabolite between the observed cross-sectional and genetic association magnitudes separately indicated only minor differences, most prominently for insulin (P diff
=0.001). Further, none of the metabolites were associated with the genetic-predisposition score if adjusting for observed BMI (data not shown).
Yet, despite the coherent association patterns, the genetic effects tended to be lower than crosssectional associations. The dashed red line in Figure 2A indicates the fit between the genetic and cross-sectional associations. The slope of 0.83±0.04 (R 2 =0.87) indicates that the genetic effects were slightly lower than anticipated from observational analyses. The slope less than unity may potentially indicate that residual confounding is inflating the observational effects. Essentially similar genetic associations magnitudes and slope were observed when analyzing an unweighted allelic score or omitting the widely studied FTO locus from the genetic-predisposition score.
Changes in adiposity and metabolic profiles
To examine the responsiveness of the metabolite profile along with spontaneous change in adiposity, we assessed associations between change in BMI and metabolite profile changes during
6-year follow-up in 1,488 young adults (Supplementary Methods). The longitudinal associations are indicated as blue half circles in Figure 1. Association magnitudes are shown as change in standardized metabolite concentration per unit increase in BMI. The metabolites most strongly associated with elevated BMI cross-sectionally also displayed the highest responsiveness to changes in BMI during the 6-year follow-up period. The association pattern of metabolite changes upon weight change largely paralleled the cross-sectional (white marks) and genetic associations
(red marks). However, the longitudinal associations were generally larger than the corresponding cross-sectional associations, as illustrated in Figure 2B. The overall correspondence between longitudinal change and cross-sectional associations fell on a straight line (R 2 =0.92) with a slope of
1.57±0.06. Deviations from the cross-sectional association pattern were observed across lipoprotein lipids, saturation of fatty acids, branched-chain amino acids as well as inflammatory markers, adiponectin and insulin. The longitudinal associations were consistent when limiting analyses to increase or decrease in BMI during the 6-year follow-up. The discrepancy between longitudinal and cross-sectional associations were even more pronounced (slope 1.73± ) when limiting analyses to individuals with baseline BMI<30 (data not shown). The results were similar when evaluated during 10-year follow-up in the same study population and replicated in an
10
independent population of middle-aged adults with 6.5-year follow-up (eFigure 4). The geneticpredisposition score was not associated with the change in BMI during follow-up (correlation r=0.009 [-0.043-0.063]).
To further illustrate the effects of weight change, the metabolite profile changes observed along modest weight loss and weight gain during 6-year follow-up are depicted in Figure 3. A weight loss of 3-6% (average 3 kg) was accompanied by substantial changes in metabolite levels throughout the metabolite profile, as indicated by the black bars in Figure 3. In contrast, a similar modest weight gain of 3-6% only displayed minor tendencies for adverse changes in the metabolite profile
(white bars). Only a considerably larger weight gain ( 6 -12%, average 7 kg, magenta bars) was
associated with a similar magnitude of metabolite changes as those observed for 3-6% weight loss.
As anticipated, weight loss was associated with favorable changes in the lipoprotein subclass profile, with decreases in VLDL and LDL concentrations. Although HDL cholesterol was not significantly altered for any of weight change categories, substantial changes were observed in the
HDL subclasses along with change in HDL particle size. Weight loss was also accompanied by a decrease in fatty acids, including PUFAs, however the ratio of PUFAs, relative to total fatty acids was increased. Further, prominent changes related to weight loss were also observed for glucose and pyruvate, which were otherwise only weakly associated with BMI in cross-sectional and genetic analyses in these young adults. While the majority of metabolite changes observed for modest weight gain were non-significant, a 6-12% weight gain did largely mirror image the metabolite changes observed for modest weight loss. In general, amino acids and numerous other cardiometabolic markers displayed substantial flexibility along with weight change, with larger differences observed for weight loss than for comparable weight gain.
COMMENT
In a large study of adolescents and young adults, elevated BMI exhibited a prominent impact throughout the comprehensive metabolite profile. The effects of genetic-predisposition to elevated BMI were concordant with a causal role of raised adiposity on the metabolite levels, even in early adulthood and within the non-obese weight range. These results demonstrate a genetic basis for the metabolic complications of obesity known as the metabolic syndrome.
(Alberti) Despite the genetic influences on the systemic metabolite measures, changes in bodyweight were
11
paralleled by changed metabolite levels to a larger extent than anticipated by causal estimates.
These findings suggest a broadly modifiable metabolite profile through weight loss for management of global cardiometabolic risk factors.
A metabolically diverse signature of elevated BMI was observed by comprehensive metabolite profiling. Although individual metabolite differences associated with one unit increment in BMI may be considered minor, the combined effects of adiposity throughout the metabolite profile render the result substantial. The majority of the assayed metabolites are known to differ between lean and obese individuals (Newgard, Pietiläinen, Jourdan) , however in this study we were able to quantify the continuous effects of elevated BMI on the metabolome in a general population setting free of comorbidities of obesity (white markers in Figure 1). Further, the application of
Mendelian randomization, utilizing a genetic-predisposition score as instrumental variable, enabled assessment of causal estimates of elevated BMI free of confounding factors and potential reverse causality (red markers in Figure 1) (DG Smith, Nordestgaard, Timpson) . The lipoprotein profile associated with elevated BMI illustrated here corroborates the established lipid pattern of obesity and highlights the heterogeneity of HDL particles (Figure1). Although LDL cholesterol is conventionally not considered as part of the atherogenic dyslipidemia cluster of the metabolic syndrome, our results indicate concurrent causal effects on IDL and particularly small LDL lipids due to elevated BMI. Further, fatty acids and their saturation were effected by normal range variation in adiposity, with the degree of omega-6 fatty acids decreased by higher adiposity. The circulating levels of omega-6 fatty acids are inversely associated with the risk for cardiovascular disease and type 2 diabetes (omega-6 paper refs, Rhee JCI) ; the causal relation with elevated BMI may contribute to underpin the cardiometabolic risk conferred by adiposity.
In addition to specific lipid signatures, a multitude of metabolites and metabolic measures were associated with BMI and illustrate the wider molecular manifestations of increased adiposity in early adulthood. The genetic estimates were consistent with a causal mediating role of elevated
BMI leading to diverse metabolic aberrations. These findings confirm the causal role of increased adiposity on elevated blood pressure (Timpson 2009) , insulin resistance (Freathy) , and low-grade inflammation (Timpson 2011) , and extends the effects to include various amino acids, liver function markers, and hormones perturbed as a consequence of excess bodyweight. Differences in branched-chain and aromatic amino acid levels between lean and obese are well-established (Felig
12
NEJM 1969, Newgard 2009) , and these amino acids have recently been shown to predict associated with the risk for development of diabetes Wang . The demonstration that genetic predisposition to elevated BMI contributes to the circulating levels could indicate that elevated concentrations of these amino acids may be considered as an intrinsic manifestations of the metabolic syndrome.
Overall, the diverse metabolic effects of genetic predisposition to elevated BMI implicate/suggest a prominent genetic basis for the risk factor clustering observed in relation to abdominal obesity and the metabolic syndrome. The genetic basis for the metabolic syndrome has proved difficult to elucidate (Kraja 2011, Kristiansson 2012) ; rather than co-occurring due to common genetic effects across components of the metabolic syndrome, our findings suggest the molecular abnormalities related to the metabolic syndrome are mediated through increased adiposity.
Eckel 2010 These results substantiate the notion of the risk for coronary heart disease related to increased BMI is conferred through intermediate metabolic risk factors.
Nordestgaard 2012 , Prospective Studies Our findings further provide evidence of the adverse metabolic effects of adiposity, not only for conventional lipids, but also for various novel biomarkers for future development of type 2 diabetes and cardiovascular disease.
With the current epidemic of obesity, the estimated 83% “spill-over effect” of elevated BMI on the metabolite profile translates into direct consequences on the cardiometabolic risk in the general population. Given the substantial heritability of BMI (Giant, Heritalibility study) , these metabolic aberrations cannot solely be attributed to life-style influences, and our results suggest an adverse effect of raised BMI irrespectively of genetic or environmental cause. Nonetheless, BMI is a major modifiable risk factor, and numerous intervention studies have demonstrated the benefit of weight loss for cardiometabolic prevention in obese individuals (USPSTF Annals, Pourier Circ
2006). Little is however known about the plasticity of the comprehensive metabolite profile with respect to spontaneous weight change in general population settings. Our longitudinal analyses of metabolite response during 6-year follow-up indicate that changes in BMI are paralleled by metabolite changes throughout the metabolome, including lipoprotein subclass levels, fatty acid composition, amino acid concentrations and metabolic markers (blue markers in Figure 1). The longitudinal associations followed a similar pattern as established in cross-sectional and genetic analyses. However, the magnitude of metabolite aberrations accompanied a 1-unit change in BMI were on average 57% greater extent than anticipated if the effects of weight change would directly translate into metabolite changes in a one-to-one relation (Figure 2). This unexpected
13
flexibility of the metabolite profile could potentially be underpinned by confounding, such as incomplete accounting for altered physical fitness Kujala , or cascading events of weight change e.g., altered insulin sensitivity with subsequent independent effects on the metabolite profile. Notably, the genetic-predisposition score was not associated with 6-year change in BMI in adulthood, and the changes are therefore likely to be primarily life-style/environmentally induced. This might indicate greater metabolic effects of acquired obesity compared to genetic factors.
(Pietiläinen?) These metabolic effects observed along with weight change further corroborate the causal adverse effect of adiposity on the systemic metabolite profile in early adulthood and irrespectively of obesity classifications.
On the positive side, the changes in the metabolite levels accompanied by a mean weight loss of
3±0.8 kg indicate that the global cardiometabolic risk profile is favorable affected by even a modest weight loss (Figure 3). The magnitudes of change in metabolite profiles were substantially larger for weight loss compared to weight gain within groups with modest weight change during the follow-up period. This contrasts the longitudinal association for the whole study population with follow-up, where magnitudes were similar for weight gain and weight loss; however, those results were derived from the whole scale of change in BMI. In addition to potential confounders, greater metabolic changes paralleled by weight loss may be attributed to the overall trends in the study populations, where 6-year changes in cholesterol levels were favorable despite an overall increase in adiposity.
Raiko 2010 The comprehensive metabolic changes concurrent with weight changes observed here may partly underpin the reduction in risk for cardiovascular disease and type 2 diabetes associated with weight loss.
(Tuomilehto NEJM, Pourier Circ 2006) Therefore, these prospective analyses emphasize the modifiable aspect of the systemic metabolite profile and highlight the public health importance of weight management in prevention of cardiometabolic diseases.
The metabolic signatures of elevated BMI were observed in generally healthy adolescents and young adults predominately within the non-obese weight range. Along with the high compatibility with causal effect estimates, these findings may suggest that even moderately elevated BMI is associated with adverse effects on the comprehensive metabolite profile and hereby at elevated risk for cardiometabolic diseases. The ideal body weight that healthy young adults should strive to attain is controversial.
de Gonzalez, Flegan 2013 Meta-analysis of standard BMI categories have suggested lower all-cause mortality for overweight compared to normal weight individuals, Flegan 2013 and in cardiovascular disease patients, overweight and obese patients have better prognosis than lean
14
patients.
(ref, JACC 2002?) The findings of the present study do not support a beneficial role of overweight on the metabolic risk factor profile, but rather suggest a causal and metabolically diverse impact of elevated BMI even in non-obese young adults and consistent effects were observed for adolescents.
Although the underlying molecular mechanisms remain elusive, both longitudinal and Mendelian randomization analyses suggest targeting BMI directly in primary prevention as an effective mean for reduction of cardiometabolic risk already in early adulthood.
Our study has both strengths and limitations. BMI is a heterogenic marker of adiposity, however it predicts the risk of related complications and is relevant for large population studies
(Prospective Studies Collaboration 2009, Emerging Risk Factors Collaboration 2011) . All results were essentially similar when adiposity was assessed by waist circumference. Our study was conducted in a homogenous population to enhance shared environmental affectors on adiposity and limit effects of population stratification on genetic analyses, however care must be taken before generalization to other ethnicities. Pleiotropy is an essential concern in Mendelian randomization, but the use of a multigenic instrument is advisable to even effects across independent loci and different molecular mechanisms.
GDS, Nordestgaard Strengths of the study include detailed metabolite profiling in large population-based cohorts of adolescents and young adults free of medication to quantify causal estimates and effects of weight change.
In conclusion, our results indicate a causal role for raised BMI with adverse effects throughout the systemic metabolite profile in apparently healthy young adults and adolescents .
Despite the genetic influence on the metabolic risk factors, and the metabolite profile was highly flexible along with weight change, and even a modest weight loss was associated with favorable changes in diverse metabolite levels. These data inform on the benefits of weight maintenance and weight loss on the systemic risk factors and motivates public health approaches to targeting
BMI as effective mean for reducing the adverse effects of the metabolic syndrome and global cardiometabolic risk.
15
ACKNOWLEDGEMENTS
This study was supported by the Academy of Finland (grant numbers 137870, 250422, 126925,
121584, 124282, 129378, 117797, and 41071, 104781, 120315, 129269, 1114194,) and the
Responding to Public Health Challenges Research Programme of the Academy of Finland (129429), the Yrjö Jahnsson Foundation, the Sigrid Juselius Foundation, the Finnish Foundation for
Cardiovascular Research, the Emil Aaltonen Foundation, the Paavo Nurmi Foundation, the Jenny and Antti Wihuri Foundation, the Juho Vainio Foundation, the Finnish Cultural Foundation, the
Social Insurance Institution of Finland, Oulu, Kuopio, Tampere and Turku University Hospital
Medical Funds, And the Medical Research Council, United Kingdom.
DISCLOSURE
No conflicts of interests declared.
16
REFERENCES
Mihalik SJ, Michaliszyn SF, de las Heras J, Bacha F, Lee S, Chace DH, DeJesus VR, Vockley J, Arslanian SA. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care. 2012 Mar;35(3):605
Moyer VA; on behalf of the U.S. Preventive Services Task Force. Screening for and Management of Obesity in Adults:
U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012 Jun 26. doi: 10.7326/0003-
4819-157-5-201209040-00475. [Epub ahead of print]
Williams PT, Krauss RM, Vranizan KM, et al.Changes in lipoprotein subfractions during diet-induced and exerciseinduced weight loss in moderately overweight men. Circulation 1990;81:1293-304.
Cheng S, Rhee EP, Larson MG, Lewis GD et al. Metabolite Profiling Identifies Pathways Associated with Metabolic Risk in Humans. Circulation 2012; 125: 2222-31.
Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, Di Angelantonio E et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 2011; 377: 1085-95.
Freathy RM, Timpson NJ, Lawlor DA, Pouta A et al. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes 2008; 57: 1419-26.
Juonala M, Magnussen CG, Berenson GS, Venn A et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. NEJM 2011; 365: 1876-85.
Finucane MM, Stevens GA, Cowan MJ et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011; 377: 557-67.
Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring Study. Arterioscler Thromb Vasc Biol 1996; 16: 1509-15.
Lee DC, Sui X, Artero EG, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on allcause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation. 2011; 124:
2483-90.
Lewis, C.E. et al. Mortality, health outcomes, and body mass index in the overweight range: a science advisory from the American Heart Association. Circulation 119, 3263–3271 (2009).
Mäntyselkä P, Kautiainen H, Saltevo j, Würtz P, Soininen P, Kangas A.J, Ala-Korpela M, Vanhala M. Weight change and lipoprotein particle concentration and particle size: a cohort study with 6.5-year follow-up. Atherosclerosis
2012; in press.
Newgard CB, An J, Bain JR et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab 2009; 9: 311-26.
Nordestgaard BG, Palmer TM, Benn M, Zacho J, Tybjærg-Hansen A, Davey Smith G, Timpson NJ. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS
Med 2012; 9: e1001212.
Peltonen M, Harald K, Männistö S, Saarikoski L, Peltomäki P, Lund L, Sundvall J, Juolevi A, Laatikainen T, Aldén-
Nieminen H, Luoto R, Jousilahti P, Salomaa V, Taimi M, Vartiainen E. The National FINRISK 2007 Study.
Publications of the National Public Health Institute, B34/2008, 72 pages.
Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H, Suomalainen A, Götz A, Suortti T, Yki-
Järvinen H, Oresic M, Kaprio J, Peltonen L. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med 2008; 5: e51.
17
Pietiläinen KH, Sysi-Aho M, Rissanen A, Seppänen-Laakso T, Yki-Järvinen H, Kaprio J, Oresic M. Acquired Obesity Is
Associated with Changes in the Serum Lipidomic Profile Independent of Genetic Effects –A Monozygotic Twin Study.
PLoS One 2007; 2:e218.
Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083-96.
Shah SH, Crosslin DR, Haynes CS, Nelson S et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012; 55: 321-30.
Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, et al. (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42: 937–948.
Wang TJ, Larson MG, Vasan RS et al. Metabolic profiles and the risk of developing diabetes. Nat Med 2011; 17: 448-53.
Würtz P, Mäkinen V-P, Soininen P et al. Metabolic signatures of insulin resistance in 7098 young adults. Diabetes.
Diabetes 2012; 61: 1372-80.
18
Figure Captions
Figure 1. Cross-sectional, genetic, and longitudinal associations of BMI with systemic metabolite
profile in young adults. Association magnitudes are indicated in standardized units of 1-SD increment in metabolite concentration per unit increment in observed BMI (white), genetic-predisposition score for BMI (red), and 6-year change in BMI (blue). Error bars denote 95% confidence intervals and highlighted arcs indicate P<0.0006. Cross-sectional and genetic associations were adjusted for sex, age, smoking status, alcohol intake and physical activity index.
Cross-sectional associations were meta-analyzed for n=15,283 adolescents and young adults using inverse-variance weighting assuming random effects. The genetic associations were scaled so that
1-unit increment in gene score corresponds to 1 kg/m 2 unit increment in observed BMI.
Results were meta-analyzed for a subset of 12,691 adolescents and young adults. Longitudinal associations of change in BMI with change in metabolite levels during 6-year follow-up were assessed for a subset of 1,488 individuals. Association magnitudes are in units of 1-SD change in metabolite concentration per unit change in BMI. Longitudinal associations were adjusted for sex, age, baseline metabolite concentration, change in smoking status, change in alcohol intake, and change in physical activity index.
Figure 2. Correspondence between genetic and longitudinal estimates with cross-sectional
associations of BMI with systemic metabolite profile. Metabolic effects of the geneticpredisposition score are compared to cross-sectional observations in Panel A, and 6-year longitudinal changes in metabolite concentrations are compared in Panel B. Shaded error bars indicate 95% confidence intervals. The red dashed line (A) indicates a linear fit of the correspondence between genetic and cross-sectional observations and circles highlighted in black denote P<0.05 for difference between associations. The dashed blue line (B) denotes the corresponding fit between longitudinal and cross-sectional associations, with highlighted squares signifying P<0.05 for difference between longitudinal and cross-sectional associations.
19
Figure 3. Metabolic changes paralleled by weight loss and weight gain. Mean changes in metabolite concentrations observed along with three categories of weight change during 6-year follow-up. Metabolite changes for 3-6% weight loss (mean (SD) loss 3.0±0.8 kg, n=119) are shown in black bars, 3-6% weight gain (3.2±0.8 kg; n=262) in white bars, and 6 -12% weight gain (7.2±1.7 kg; n=184) in magenta. Error bars indicate 95% confidence intervals. Metabolite concentrations were adjusted for age, sex, current smoking, alcohol intake and physical activity index at baseline and follow-up. The 6-year differences in metabolite levels are shown in units of 1-SD baseline metabolite concentration.
20
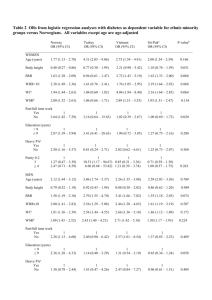
Table 1. Characteristics of the study populations.
Clinical characteristics
Participants (men/women)
Age [yr]
BMI
NFBC1986
5546
(2793/2753)
16 (-)
21.2 (3.4)
Waist circumference [cm]
Waist/Hip ratio
Systolic blood pressure [mmHg]
Total cholesterol [mmol/L]
HDL cholesterol [mmol/L]
Triglycerides [mmol/L]
Glucose [mmol/L]
Insulin [mU/L]
Physical activity index
Alcohol usage [g/day]
Smoking prevalence [%]
Prevalence of overweight [%]
Prevalence of obesity [%]
74 (9)
0.80 (0.06)
116 (13)
4.3 (0.8)
1.4 (0.3)
0.7 [0.6-1.0]
5.2 [4.9-5.4]
9.6 [7.4-12]
30 [18-43]
-
11 (11-12)
9 (8-10)
3 (2-3)
Increment in BMI per unit of genetic-predisposition score [kg/m 2 ]
Proportion of BMI explained
0.91±0.10
P=8e-21
2.2%
NFBC1966
5225
(2621/2604)
31 (-)
24.6 (4.0)
84 (12)
0.86 (0.08)
125 (13)
5.0 (1.0)
1.6 (0.4)
1.0 [0.7-1.4]
5.0 [4.7-5.3]
7.5 [6.1-9.4]
11 [4-21]
4 [1-11]
40 (39-41)
31 (30-32)
8 (8-9)
1.21±0.11
P=1e-28
2.6%
YFS
2367
(1104/1263)
31.9 (4.9)
25.0 (4.4)
84 (12)
0.84 (0.08)
117 (13)
5.1 (0.9)
1.3 (0.3)
1.1 [0.8-1.6]
5.0 [4.7-5.3]
6 [5-9]
13 [3-31]
5 [0-15]
24 (22-26)
32 (30-33)
12 (11-13)
0.91±0.17
P=1e-7
1.3%
FINRISK 1997
2145
(994/1151)
32.3 (4.5)
24.7 (4.0)
82 (12)
0.82 (0.08)
125 (14)
5.0 (1.0)
1.4 (0.3)
1.0 [0.8-1.4]
4.8 [4.5-5.1]
4.6 [3.3-6.5]
-
4 [0-11]
28 (26-30)
32 (30-34)
9 (8-10)
1.22±0.25
P=1e-6
1.4%
Values are mean (SD) or median [interquartile range] or percentage (95% confidence interval) for normally distributed, skewed and categorical variables, respectively. *Physical activity-index was not assessed in FINRISK. The increment in BMI per unit of genetic-predispostion score was based on weighting each genetic variant in the score by effects established in large-scale meta-analysis.
Giant2010 The genetic-predisposition score was subsequently scaled to exactly 1 kg/m 2 for each cohort prior to estimating effects on the metabolite profile.
21
Figure 1.
22
Figure 2.
23
Figure 3.
24
Supplementary Online Content
Würtz P, Wang Q, Kangas AJ, et al. Metabolic Signatures of Adiposity and Weight
Change in Young Adults: Observational and Genetic Evidence. eMethods: Study characteristics, laboratory and genotyping assays.
eTable 1. Mean metabolite concentrations for men and women in each study population.
eFigure 1: Mendelian Randomization Framework.
eFigure 2: Inter-correlations of the assayed metabolites.
eFigure 3: Cross-sectional metabolite associations with BMI for men and women in each cohort.
eFigure 4: Consistency of longitudinal changes metabolite concentrations with changes in BMI across follow-up time and in an independent study population.
This supplementary material has been provided by the authors to give readers additional information about their work.
25
eMethods: Study characteristics, biomarkers and genotyping assays.
Study populations
The Northern Finland Birth Cohorts (NFBC) of 1986 and 1966 were initiated to study factors affecting preterm birth and subsequent morbidity in the two Northernmost provinces in Finland
(http://http://kelo.oulu.fi/NFBC). For NFBC-1986, the number of deliveries in the birth cohort was
9,362, which was 99% of all the deliveries taking place in the target period of the cohort. Data collection in 2001-2002 included clinical examination and serum sampling at age 15-16 for 6,621 adolescent boys and girls; data from this time point were used for the present study.
Kantomaa2012,
Veltsista 2010 Attendees in the 16-year field study (71%) were adequately representative of the original cohorts (Järvelin 2004, Veltsista 2010). In total, 5,602 adolescents had a systemic metabolite profile measured, of which 95% of the serum samples were drawn after overnight fasting. The NFBC-1966 included 12,058 children born into the cohort, comprising 96% of all births during 1966 in the region. Data collection in 1997 included clinical examination and blood serum sampling at age 31 for 6,007 individuals. Data from this time point were used for the present study. Attendees in the 31-year field study (52% of target population) were representative of the original cohort (Järvelin 2004). In total, 5,709 persons had their metabolite profile quantified, of which 96% were based on fasting serum samples.
For both NFBC studies, blood pressure was measured using a mercury sphygmomanometer from the right arm after 15 minutes rest. The average of two readings was used for the analyses.
Physical activity index was calculated as metabolic-equivalent-of-task, based on based on questionnaire data on frequency, intensity, and duration of physical activity as described previously.(Kantomaa 2012, Würtz Diabetes 2012). Current smoking and alcohol usage
(doses/day) were assessed from questionnaires, with alcohol consumption assumed zero in NFBC-
1986. Medication for hypertension, diabetes and hypercholesterolemia was also assessed by questionnaires. In addition to NMR-based metabolite profiling, circulating levels of the following biomarkers were measured: C-reactive protein, alanine aminotransferase, gamma-glutamyl aminotransferase, bilirubin, and insulin by standard clinical assays (Liver GWAS, Würtz Diabetes).
Testosterone and sex-hormone binding globulin were measured by mass spectrometry (ref). For
NFBC-1966 also vitamin-D levels were measured. Informed consent was obtained from all participants, and the research protocols were approved by the Ethics Committee of Northern
Ostrobotnia Hospital District, Finland.
The Cardiovascular Risk in Young Finns Study was designed to study associations of childhood risk factors to cardiovascular disease in adulthood (http://med.utu.fi/cardio/youngfinnsstudy/). The baseline study in 1980 included 3,596 children and adolescents aged 3 to 18. Data in the crosssectional analyses are from 2001 field study, which included 2,247 individuals with an overnight fasting metabolomics profile (response rate 63%). These individuals were representative of the baseline cohort (Raitakari 2008). Longitudinal data on BMI change and change in metabolite levels were collected for 1,488 individuals who participated in the 2007 field study and were free of medication for metabolic diseases also at 6-year follow-up. The longitudinal associations were further assessed for consistency over 10-year (2001-2011; n=1,529) follow-up. Blood pressure was measured using a random-zero sphygmomanometer, the average of the three measurements were taken. Metabolic-equivalent-of-task was used as physical activity index. Current smoking, alcohol usage and medication for hypertension, diabetes and hypercholesterolemia were assessed by questionnaires. In addition to high-throughput metabolite profiling, the following biomarkers were assayed and analyzed in the present study: homocysteine, C-reactive protein, phospholipase
26
activity, alanine aminotransferase, gamma-glutamyl aminotransferase, leptin, adiponectin, vitamin
D, and insulin by standard clinical assays (Liver GWAS, Würtz Diabetes, Raitakari 2008).
Testosterone and sex-hormone binding globulin were measured by mass spectrometry for both en and women in 2001, but only for men in 2007. Participants gave written informed consent, and the study was approved by the local Ethics Committees of the study sites.
The FINRISK 1997 study was conducted to monitor the health of the Finnish population among persons aged 24–74 at recruitment. In total, 8,444 individuals were recruited to represent the general population of the study areas. Metabolite profiling from non-fasting serum samples were measured for 7,503 individuals. The median fasting time was 5h (interquartile range 4-6h). Only individuals under the age of 40 were included the main analyses of the present study. The generalizability of the results to older ages was examined separately for persons aged above 40
(eFigure 2). Blood pressure measures were collected and participants filled questionnaires of physical activity. Participants filled questionnaires of smoking status, alcohol usage, physical activity and medication. In the absence of sufficient information to derive metabolic-equivalentof-task, a binary physical activity index was created based on the self-reported level of leisure time physical activity. In addition to high-throughput metabolite profiling, the following biomarkers were assayed and analyzed in the present study: homocysteine, C-reactive protein, phospholipase activity, gamma-glutamyl aminotransferase, leptin, adiponectin, and insulin by standard clinical assays (Blankenberg Circ 2010). Testosterone and sex-hormone binding globulin ?. Participants gave written informed consent and the FINRISK study was approved by the ethical committee of the National Public Health Institute, Helsinki, Finland.
Consistency of cross-sectional and longitudinal associations in older persons
The generalizability of the observational results was examined in the Pieksamaki population cohort (Würtz 2012). Short description.
All metabolites and biomarkers were standardized to z-scores to enable comparison across potentially different assays.
27
Genotyping
Genotyping was conducted on HumanHap 370k (Infinium 370cnvDuo) platform for NFBC-1966
(n=4,671) and 670k Illumina platforms for YFS (n=2,171). Variants not directly genotyped were imputed based on HapMap 2. The 32 genetic variants constituting the gene score were directly genotyped in NFBC-1986 by SNPlex (Speliotes Giant-2010). The allelic correlates with BMI were combined to genetic-predisposing score by summing the allele count for each individual weighted by the previously determined effect of BMI. For FINRISK 1997, a subset of 20 genetic variants were genotyped by iPLEXTM Sequenom MassARRAY. Specifically, the BMI-correlated variants (Giant) in the following loci were examined: MC4R, BDNF, SH2B1, ETV5, RBJ, GPRC5B, MAP2K5, QPCTL, TNNI3K,
SLC39A8, FLJ35779, LRRN6C, TMEM160, FANCL, PRKD1, LRP1B, PTBP2, MTIF3, ZNF608, NUDT3.
The genetic-predisposition score in FINRISK was calculated as the weighted allele count of the listed variants and scaled to be associated exactly with 1 kg/m 2 per increment in the gene score.
Descriptive data for all 32 genetic variants are given eTable 2.
Metabolite quantification
Total lipid concentrations in fourteen lipoprotein subclasses were calibrated via high-performance liquid chromatography. The lipoprotein subclasses sizes were defined as follows: extremely-large
VLDL (particle diameter from 75 nm upwards), five VLDL subclasses (average particle diameters of
64.0 nm, 53.6 nm, 44.5 nm, 36.8 nm, and 31.3 nm), IDL (28.6 nm), three LDL subclasses (25.5 nm,
23.0 nm, and 18.7 nm), and four HDL subclasses (14.3 nm, 12.1 nm, 10.9 nm, and 8.7 nm).
Metabolite quantification of lipid particle constituents and small molecules were conducted by regression modeling as described previously.
Soininen, Inouye
28
eTable 1: Mean metabolite concentrations for men and women in each study population.
Yellow marks different assays: to be checked.
Metabolite
Extremely large VLDL
[mmol/L]
Very large VLDL
[mmol/L]
Large VLDL [mmol/L]
Medium VLDL
[mmol/L]
Small VLDL [mmol/L]
Very small VLDL
[mmol/L]
IDL [mmol/L]
Large LDL [mmol/L]
Medium LDL [mmol/L]
Small LDL [mmol/L]
Very large HDL
[mmol/L]
Large HDL [mmol/L]
Medium HDL [mmol/L]
Small HDL [mmol/L]
VLDL particle size [nm]
LDL particle size [nm]
HDL particle size [nm]
Total cholesterol
[mmol/L]
Non-HDL cholesterol
[mmol/L]
VLDL cholesterol
[mmol/L]
IDL cholesterol
[mmol/L]
LDL cholesterol
[mmol/L]
HDL cholesterol
[mmol/L]
Cholesterol esterification % [%]
Apolipoprotein B [g/l]
Apolipoprotein A1 [g/l]
Triglycerides [mmol/L]
VLDL Triglycerides
[mmol/L]
Total fatty acids
[mmol/L] n-3 fatty acids
[mmol/L] n-3 fatty acids/ total fatty acids [%] n-6 fatty acids
[mmol/L] n-6 fatty acids
NFBC-1986
0.007
[0.0022-0.016]
0.032
[0.016-0.060]
0.13 [0.072-0.24]
0.35 [0.24-0.52]
0.48 [0.38-0.60]
0.40 [0.34-0.47]
0.93 [0.79-1.1]
1.1 [0.95-1.3]
0.64 [0.54-0.77]
0.40 [0.32-0.48]
0.36 [0.25-0.49]
0.73 [0.56-0.93]
0.82 [0.72-0.94]
1.1 [0.98-1.1]
36 [36-37]
24 [23-24]
9.9 [9.8-10]
4.1 [3.6-4.6]
2.7 [2.3-3.1]
0.58 [0.46-0.71]
0.61 [0.52-0.71]
1.5 [1.2-1.8]
1.4 [1.2-1.6]
0.72 [0.72-0.73]
0.75 [0.66-0.88]
1.5 [1.3-1.6]
0.86 [0.67-1.1]
0.52 [0.36-0.75]
9.1 [8.1-10]
0.32 [0.27-0.37]
3.5 [3.1-3.9]
3.1 [2.8-3.5]
34 [32-36]
NFBC-1966 YFS
8.3e-3 [2.0e-3-0.020] 7.4e-3 [8.9e-4-0.023]
0.029 [0.011-0.064] 0.031 [8.8e-3-0.077]
0.13 [0.060-0.27] 0.17 [0.091-0.34]
0.39 [0.26-0.62]
0.60 [0.46-0.78]
0.50 [0.40-0.61]
1.2 [0.97-1.4]
1.5 [1.2-1.8]
0.87 [0.72-1.1]
0.54 [0.44-0.67]
0.44 [0.31-0.59]
0.79 [0.54-1.1]
0.88 [0.74-1.1]
1.2 [1.1-1.3]
36 [35-37]
24 [23-24]
9.9 [9.7-10]
5.1 [4.5-5.9]
3.5 [2.9-4.2]
0.71 [0.56-0.92]
0.76 [0.65-0.91]
2.0 [1.6-2.4]
1.6 [1.4-1.9]
0.74 [0.73-0.75]
0.94 [0.80-1.1]
1.7 [1.5-1.9]
1.0 [0.74-1.4]
0.57 [0.37-0.89]
11 [9.3-13]
0.37 [0.30-0.48]
3.3 [2.8-4.0]
3.8 [3.3-4.4]
35 [33-37]
0.47 [0.32-0.71]
0.58 [0.45-0.76]
0.49 [0.40-0.59]
1.2 [0.98-1.4]
1.4 [1.2-1.7]
0.85 [0.70-1.0]
0.54 [0.44-0.65]
0.33 [0.21-0.48]
0.74 [0.49-1.0]
1.0 [0.88-1.1]
1.2 [1.1-1.3]
36 [36-37]
24 [23-24]
9.9 [9.7-10]
4.9 [4.3-5.6]
3.3 [2.7-3.9]
0.64 [0.49-0.82]
0.72 [0.61-0.85]
1.9 [1.6-2.3]
1.5 [1.3-1.8]
0.72 [0.71-0.73]
0.92 [0.77-1.1]
1.7 [1.5-1.8]
1.1 [0.87-1.6]
0.69 [0.46-1.0]
10 [9.0-12]
0.36 [0.29-0.45]
3.4 [2.9-4.0]
3.5 [3.1-4.0]
34 [32-37]
FINRISK 1997
0.015
[7.6e-3-0.030]
0.029
[0.013-0.062]
0.097 [0.037-0.20]
0.28 [0.17-0.43]
0.53 [0.40-0.68]
0.51 [0.41-0.63]
1.1 [0.95-1.3]
1.3 [1.1-1.6]
0.79 [0.64-0.96]
0.51 [0.42-0.63]
0.68 [0.54-0.87]
0.88 [0.69-1.1]
0.72 [0.59-0.87]
1.0 [0.92-1.1]
35 [35-36]
23 [23-24]
10 [10-10]
4.9 [4.3-5.5]
3.2 [2.6-3.9]
0.68 [0.54-0.85]
0.73 [0.62-0.86]
1.8 [1.5-2.2]
1.6 [1.4-1.8]
0.77 [0.75-0.79]
0.88 [0.75-1.0]
1.7 [1.5-1.8]
0.94 [0.70-1.3]
0.43 [0.27-0.65]
11 [9.1-12]
0.39 [0.32-0.48]
3.6 [3.2-4.2]
3.7 [3.3-4.3]
35 [33-38]
29
total fatty acids [%]
MUFA [mmol/L]
MUFA/ total fatty acids [%]
PUFA [mmol/L]
PUFA/ total fatty acids [%]
Saturated fatty acids
[mmol/L]
Saturated fatty acids/ total fatty acids [%]
Docosahexaenoic acid
[mmol/L]
Linoleic acid [mmol/L]
Double bonds/
Fatty acid [%]
Methylene groups/
Fatty acid [%]
Fatty acid chain length
Phosphoglycerides
[mmol/L]
Phosphatidylcholines
[mmol/L]
Sphingomyelin
[mmol/L]
Glucose [mmol/L]
Lactate [mmol/L]
Pyruvate [mmol/L]
Citrate [mmol/L]
Glycerol [mmol/L]
Alanine [mmol/L]
Glutamine [mmol/L]
Glycine [mmol/L]
Histidine [mmol/L]
2.6 [2.2-3.1]
0.29 [0.27-0.31]
3.4 [3.1-3.8]
0.38 [0.36-0.39]
3.1 [2.7-3.5]
0.34 [0.33-0.35]
0.12 [0.10-0.15]
2.6 [2.3-2.9]
1.2 [1.2-1.3]
9.8 [9.7-9.8]
18 [18-18]
0.69 [0.61-0.79]
1.6 [1.4-1.9]
0.23 [0.21-0.26]
4.5 [4.2-4.8]
1.4 [1.2-1.7]
0.072 [0.061-0.085]
0.10 [0.089-0.12]
0.063 [0.049-0.081]
3.0 [2.5-3.8]
0.28 [0.26-0.30]
4.2 [3.7-4.9]
0.39 [0.36-0.41]
3.6 [3.1-4.4]
0.33 [0.32-0.35]
0.16 [0.12-0.21]
3.2 [2.8-3.7]
1.3 [1.2-1.3]
9.8 [9.7-9.9]
18 [18-18]
0.86 [0.74-1.0]
2.0 [1.8-2.4]
0.35 [0.31-0.41]
4.6 [4.3-5.1]
1.5 [1.2-1.8]
0.085 [0.071-0.10]
0.10 [0.093-0.12]
0.081 [0.063-0.11]
3.1 [2.6-3.8]
0.30 [0.28-0.32]
3.9 [3.5-4.4]
0.38 [0.35-0.40]
3.3 [2.9-4.0]
0.32 [0.31-0.34]
0.16 [0.12-0.20]
3.0 [2.7-3.5]
1.3 [1.2-1.3]
9.7 [9.6-9.8]
18 [18-18]
0.83 [0.73-0.96]
2.0 [1.8-2.3]
0.29 [0.25-0.33]
4.8 [4.6-5.2]
1.5 [1.3-1.7]
0.11 [0.098-0.13]
0.084 [0.065-0.11]
2.9 [2.4-3.5]
0.28 [0.26-0.30]
4.2 [3.6-4.7]
0.39 [0.37-0.42]
3.5 [2.9-4.2]
0.33 [0.31-0.35]
0.16 [0.12-0.20]
3.1 [2.8-3.6]
1.3 [1.2-1.3]
9.5 [9.4-9.7]
18 [18-18]
0.74 [0.63-0.86]
1.8 [1.5-2.0]
0.26 [0.22-0.30]
4.3 [4.0-4.6]
1.2 [1.0-1.4]
0.080 [0.066-0.10] 0.069 [0.059-0.081]
0.11 [0.095-0.12]
0.11 [0.090-0.15]
0.40 [0.36-0.44]
0.52 [0.48-0.57]
0.43 [0.38-0.48]
0.54 [0.49-0.60]
0.42 [0.37-0.47]
0.54 [0.48-0.59]
0.41 [0.37-0.45]
0.47 [0.43-0.52]
0.28 [0.25-0.31] 0.32 [0.28-0.36] 0.30 [0.27-0.34] 0.30 [0.27-0.34]
0.064 [0.058-0.071] 0.068 [0.062-0.077] 0.071 [0.064-0.079] 0.065 [0.059-0.071]
Homocysteine [µmol/l]
Isoleucine [mmol/L]
Leucine [mmol/L]
Valine [mmol/L]
Phenylalanine
[mmol/L]
0.082 [0.073-0.092] 0.088 [0.077-0.10]
0.20 [0.18-0.23] 0.22 [0.19-0.25]
0.086 [0.075-0.10]
0.21 [0.19-0.25]
0.081 [0.070-0.093]
0.19 [0.17-0.22]
Tyrosine [mmol/L]
Acetate [mmol/L]
0.069 [0.063-0.076] 0.083 [0.074-0.094] 0.077 [0.069-0.085] 0.077 [0.070-0.086]
0.051 [0.044-0.058] 0.053 [0.045-0.062] 0.056 [0.048-0.064] 0.047 [0.040-0.055]
0.045 [0.040-0.052] 0.041 [0.036-0.048] 0.041 [0.035-0.050] 0.048 [0.041-0.055]
Acetoacetate [mmol/L] 0.036 [0.026-0.057] 0.042 [0.030-0.065] 0.038 [0.028-0.056] 0.059 [0.040-0.094] beta-hydroxybutyrate
[mmol/L]
Creatinine [mmol/L]
Urea [mmol/L]
- - 9.2 [7.8-11] 10 [8.8-12]
0.053 [0.046-0.062] 0.052 [0.043-0.064] 0.056 [0.047-0.068] 0.051 [0.043-0.064]
0.099 [0.078-0.15]
0.054 [0.048-0.061] 0.064 [0.056-0.073] 0.065 [0.057-0.074] 0.056 [0.048-0.064]
0.046 [0.031-0.062]
0.12 [0.092-0.17]
0.055 [0.038-0.075]
0.081 [0.065-0.11]
0.075 [0.052-0.098]
0.17 [0.11-0.29]
0.045 [0.030-0.061]
Albumin [cu]
C-reactive protein
[mg/L]
Phospholipase Activity
[nmol/ml/min]
Alpha-1-acid glycoprotein [cu]
0.098 [0.093-0.10]
0.12 [0.048-0.47]
-
1.3 [1.2-1.4]
0.10 [0.094-0.11]
0.71 [0.35-1.7]
-
1.3 [1.2-1.5]
0.10 [0.098-0.11]
0.72 [0.33-1.8]
1.5 [1.2-1.9]
1.3 [1.2-1.5]
0.097 [0.094-0.10]
0.76 [0.38-1.7]
230 [190-270]
1.3 [1.2-1.4]
30
Alanine aminotransferase [U/L]
γ-glutamine aminotransferase [U/L]
Bilirubin [µmol/L]
Leptin [ng/ml]
Adiponectin [µg/mL]
Systolic blood pressure
[mmHg]
Diastolic blood pressure [mmHg]
Testosterone (Men)
[nmol/l]
Testosterone (Women)
[nmol/l]
SHBG (Men) [nmol/l]
SHBG (Women)
[nmol/l]
Vitamin D [nmol/l]
Insulin [IU/L]
10 [7.0-13]
13 [10-17]
8.7 [6.4-12]
-
-
115 [107-124]
68 [63-73]
20 [16-25]
1.6 [1.3-2.1]
30 [22-38]
52.1 [36.9-75.7]
-
9.6 [7.4-12]
8.0 [6.0-11]
15 [10-24]
3.0 [2.0-5.0]
-
-
124 [115-133]
77 [70-85]
21 [17-26]
1.0 [0.7-1.3]
32 [24-41]
57.7 [42.2-78.4]
19 [15-23]
7.5 [6.1-9.4]
15 [11-22]
18 [13-29]
-
7.7 [4.1-14]
8.6 [6.1-12]
116 [108-125]
71 [64-78]
18 [15-22]
1.4 [1.1-1.9]
29 [23-37]
61 [42-101]
43 [33-54]
6.0 [5.0-9.0]
-
20 [15-29]
-
6.6 [3.2-13]
5.5 [3.6-8.3]
124 [115-134]
76 [70-83]
-
-
-
-
-
4.6 [3.3-6.5]
31
eFigure 1: Mendelian Randomization Framework.
Although GRS-metabolite effect sizes were modest, the overall association pattern was consistent with the 1.8% variance in BMI explained by the GRS. As illustrated in Figure 3B, the expected effect size of the GRS on the metabolites was modeled through a Mendelian triangulation approach
(Freathy 2008, Sheehan 2008), where GRS-metabolite associations were hypothesized to be mediated through BMI and hereby reflect the epidemiological BMI-metabolite associations observed in this study. In particular, if a GRS-metabolite association (c; shown in Figure 3A) is mediated through BMI, then the expected effect size would be c = a x b, where a is the GRS-BMI association (a=1.07, Figure 3B) and b is the observed BMI-metabolite associations (Figure 1).
Following this hypothesis, the GRS-metabolite associations were largely consistent, albeit somewhat smaller than the excepted effects (based on the epidemiological observations) as depicted in Figure 3C. For example, the genetic effect size on HDL diameter was β=-0.065 SD/[kg m -2 ] whereas the expected effect size was c=1.07×-0.068=-0.073 SD/[kg m -2 ].
32
eFigure 2: Correlations of the assayed metabolites.
The colour coding indicates Pearson’s correlation coefficients in the current study (n= 15,283 ). The correlations were analyzed separately for each of the four cohorts and combined using inversevariance weighted meta-analysis.
33
eFigure 3. Cross-sectional metabolite associations with BMI for men and women in each cohort.
To be updated: FinRiskOldies and Pieksamaki can go to same plot. Just show 95% CI, with minimal marking of exact association estimate.
Show men and women combined or separately?
34
eFigure 4. Consistency of longitudinal changes metabolite concentrations with changes in BMI across follow-up time and in an independent study population.
References:
35