Electrochemical properties of RDS NiO in the sensitized state
advertisement
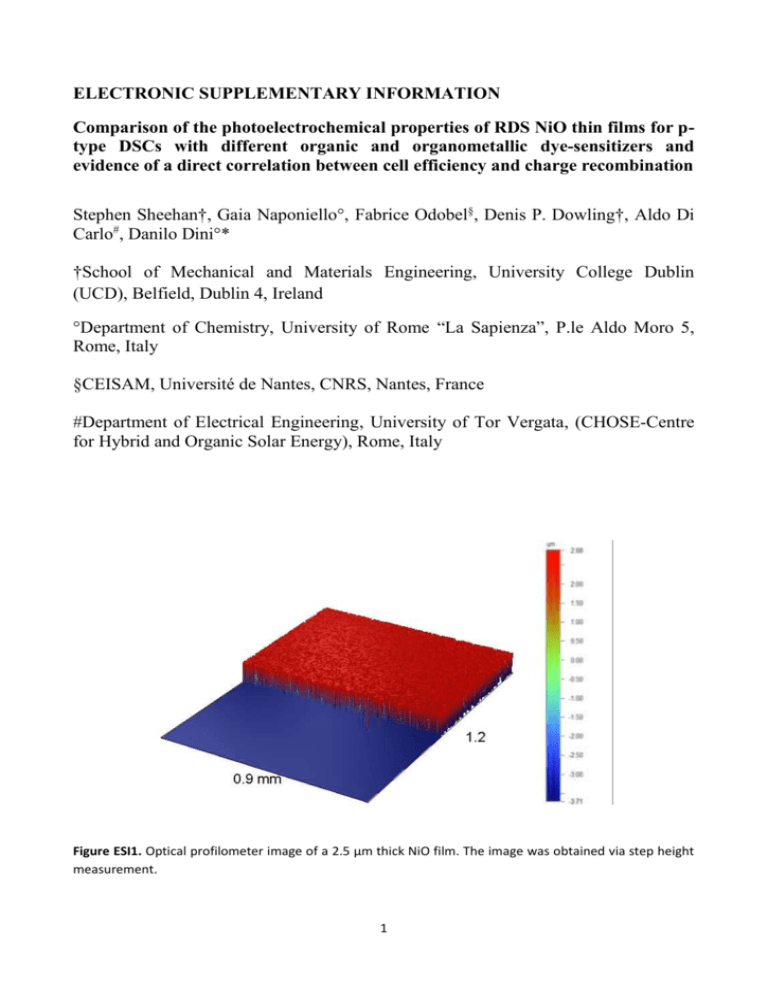
ELECTRONIC SUPPLEMENTARY INFORMATION Comparison of the photoelectrochemical properties of RDS NiO thin films for ptype DSCs with different organic and organometallic dye-sensitizers and evidence of a direct correlation between cell efficiency and charge recombination Stephen Sheehan†, Gaia Naponiello°, Fabrice Odobel§, Denis P. Dowling†, Aldo Di Carlo#, Danilo Dini°* †School of Mechanical and Materials Engineering, University College Dublin (UCD), Belfield, Dublin 4, Ireland °Department of Chemistry, University of Rome “La Sapienza”, P.le Aldo Moro 5, Rome, Italy §CEISAM, Université de Nantes, CNRS, Nantes, France #Department of Electrical Engineering, University of Tor Vergata, (CHOSE-Centre for Hybrid and Organic Solar Energy), Rome, Italy Figure ESI1. Optical profilometer image of a 2.5 µm thick NiO film. The image was obtained via step height measurement. 1 Figure ESI2. Absorption spectrum of bare RDS NiO (l = 0.4 m) deposited onto FTO/glass. Air was taken as blank. Electrochemical properties of RDS NiO in the bare state The electrochemical properties of a bare NiO electrode were analysed using cyclic voltammetry in a redoxfree electrolyte. The voltammogram of bare nanoporous NiO electrode recorded in non aqueous electrolyte (Figure ESI3) showed features similar to those of RDS NiO voltammograms recorded in aqueous medium.[20] Figure ESI3. Voltammogram of NiO deposited onto FTO via MW plasma sintering. Scan rate 5 mV/s, electrolyte 0.2 M LiClO4 in MPN (configuration of three electrode cell with Ag/AgCl as reference electrode and Pt as counter electrode). The two characteristic peaks of NiO oxidation have been indicated in the voltammogram. 2 Current waves are generated by the reversible transformations of Ni(II) to Ni(III) (anodic peak I centered at about 0.3 V vs Ag/AgCl) and of Ni (III) to Ni(IV) (anodic peak II centered at about 0.9 vs Ag/AgCl),[ESI1] with the involvement of ClO4- as anion responsible for the compensation of the anodic charge on the surface of NiO (Eqs. 1 and 2). NiO + m ClO4- → Ni(II)1-mONi(III)m(ClO4)m + m e− Equation 1 for anodic peak I, and Ni(II)1-mONi(III)m(ClO4)m + m ClO4- → Ni(II)1-mONi(IV)m(ClO4)2m + m e− Equation 2 for anodic peak II (Figure ESI3) with the perchlorate anion behaving as charge compensating species.[ESI1] Through the analysis of the XPS profiles of oxidized NiO when the electrolyte is 0.2 M LiClO4 in 3-MPN it has been proved the involvement of ClO4– as charge compensating species.[ESI2] Evidence of that is given in the O 1s XP spectra (not shown) of oxidized mesoporous NiO which present the typical signals arising from the O atoms belonging to perchlorate anions that have been chemisorbed on the surface of NiO during the process of NiO oxidation (Eqs. 1 and 2).[ESI3] The presence of ClO4- anions in oxidized NiO was detected also in the Cl 2p XPS region of a NiO sample polarized at 0.5 and 0.85 V vs AgAg/Cl.[ESI2] Unlike the case reported in ref. 33, the participation of Li+ cation (from the electrolyte) in the mechanism of charge compensation for NiO oxidation in non-aqueous electrolyte was not relevant. The reason for this difference relies on the fact that here we have used an anion (ClO4-) with a much larger transport number with respect to triflate utilized in the work of ref.33. An anion like triflate is a quasi immobile species which absorbs on electrodes: its employment generates a situation in which the cation is practically the sole species that carries the ionic current through the electrolyte.[ESI4] This implies that the mechanisms of charge compensation (either anodic or cathodic) at those electrodes that vary their oxidation state will be mainly based on the exchange of cations when triflate is the electrolyte anion. The features observed in ref. 33 are a consequence of the nature of electrolyte cation because of the lack of any involvement of a large anion like triflate in the mechanisms of charge compensation. In the present case perchlorate anion is a much 3 more mobile species than triflate with consequent involvement of the former anion in the charge compensation mechanisms of oxidized NiO. Figure ESI4 shows the voltammograms of NiO in non aqueous electrolyte recorded at different scan rates. Figure ESI4. Voltammograms of bare NiO at different scan rates in 0.2M LiClO4 MPN. The dependence of the oxidation peaks of nanoporous NiO is generally linear with the scan rate of the voltammetry (Figure ESI5). Such a dependence corresponds to the occurrence of a surface-confined redox process,[ESI4] the rate of which scarcely depends on the diffusion of charge carriers. This is consistent with the data presented by Boschloo et al. in aqueous electrolyte.[33] The slight displacement of the oxidation peaks with scan rate consists in a positive shift of the oxidation peaks upon increase of scan rate (Figure ESI4). This effect is related with the diffusive transport phenomena that become progressively important in the kinetics of NiO oxidation process with increasing scan rate.[ESI4] The transport phenomena involved in the process of NiO oxidation are the diffusion of charge compensating ClO4- anions from the bulk of the electrolyte to the NiO/electrolyte interface, and, eventually, diffusion of ClO4- anions either on NiO surface 4 or within oxide structure through solid defects, e.g. grain boundaries, or discontinuities like Van der Waals planes, to compensate the charge of oxidized NiO.[ESI5] The multiple oxidation of NiO (Eqs.1 and 2) with kinetic features typical of surface confined processes (vide supra),[ESI4] corresponds to the electrochemical injection of holes in dark conditions.[4c,20] Figure ESI5. Linear dependence of the amplitude of the second anodic and the second cathodic peak of bare NiO with scan rate. Data extracted from Figure ESI4. The presence of the redox couple I-/I3- in the electrolyte, introduces a new strong current of oxidation due the process 3 I- → I3- + 2e- (Figure ESI6), which follows the dark oxidation of NiO (Eqs.1 and 2, Figures ESI3 and ESI6). 5 Figure ESI6. Dark voltammogram of NiO with and without redox shuttle. Scan rate: 20 mV s -1. Electrolyte compositions were 0.2 M LiClO4 in MPN (red curve) and 0.2 M I-/0.02 M I2 in MPN (violet curve). Configuration was a three-electrode cell with Ag/AgCl as reference electrode, and Pt as counter electrode. The comparison of the voltammograms in Figure ESI6 shows that the oxidation of iodide involves a much larger current with respect to the process based solely on NiO oxidation (Figure ESI3). Since the dark oxidation of iodide starts only at the condition that NiO gets first oxidized (Eq.1), the layer of mesoporous NiO in the oxidized state acts as an electrocatalyst towards the charge recombination process of iodide oxidation. The eventual shunting due to the oxidation of iodide directly on uncovered FTO does not seem to be important when the layer of mesoporous NiO is present with a thickness of 0.3 - 4 m. This is because iodide oxidation on bare FTO occurs at much larger potential values with respect to bare RDS NiO (Figure ESI7). Beside the electrocatalytic effect of NiO towards the dark oxidation of iodide, RDS NiO operates also with a much larger surface area with respect to the TCO made of FTO in the type of sample here considered (Figure 1). This combination of findings indicates that the process of charge recombination based on the oxidation of I- (as the redox species) on mesoporous RDS NiO (as semiconducting electrode) is mainly located at the surface of the metal oxide and it is activated by the injection of holes in NiO. 6 Figure ESI7. Dark voltammogram of NiO/FTO (violet curve) and bare FTO (black curve) with redox shuttle. Scan rate: 20 mV/s. Electrolyte composition: 0.2 M I-/0.02 M I2 in MPN. Configuration was a three-electrode cell with Ag/AgCl as reference electrode and Pt as counter electrode. Electronic properties of sensitizers and spectra of sensitized NiO Figure ESI8. Structure of the four dyes utilized in this work for the sensitization of RDS NiO cathodes. The frontier energy levels of P1, SQ2, N719, BD and NiO show that the HOMO and LUMO levels of the four dyes match respectively with the upper edge of NiO VB and the Nernst level of the couple I-/I3- (Figure ESI9). 7 Therefore, the photoactivated transfer of the hole from NiO to the excited anchored dye and the successive transfer of the electron from the excited (or temporarily reduced) [4a] dye to the oxidized form of the redox couple are both allowed in terms of energy when NiO is sensitized with P1, SQ2, N719 and BD. Figure ESI9. Energy band diagram of NiO and HOMO/LUMO levels of N719, BD, SQ2 and P1 sensitizers. The redox level of the couple iodide/triiodide is also shown for reference. Figure ESI10. Absorption spectra for sensitized NiO (l = 1 m) using N719, SQ2 and BD as dyes. Air was taken as blank. 8 Figure ESI11. Absorption spectra of bare RDS NiO (l = 2.5 m) and P1-sensitized RDS NiO deposited onto FTO/glass. Air was taken as blank. Electrochemical properties of RDS NiO in the sensitized state The presence of the sensitizer induces generally an effect of passivation for the NiO layer towards its oxidation, i.e. its dark hole injection (Eqs.1 and 2). Such an effect is observed through the decrease of the intensity of the current peaks I and II (Figure ESI3) when NiO passes from bare to the sensitized state (Figure ESI12). Therefore, the dark current profiles determined with the sensitized samples can be ascribed to the same electrochemical processes of the bare oxide according to Eqs. 1 and 2. NiO electrodes were sensitized in the following solutions of the dye: N719 (3 10-4 M), BD (3 10-4 M) and SQ2 (1 10-3 M) in a pure ethanol solution, and P1 (2.5 10-3 M) in acetonitrile with a dipping time of 2 h. The most important feature of the process of oxidation of NiO in the sensitized state is the absence of redox peaks ascribable to the oxidation of the anchored dye in case of P1, BD and N719 sensitizers. These findings on NiO sensitized with P1, BD and N719 resemble the electrochemical behaviour of RDS NiO sensitized with erythrosine B.[4c] Only in the case of NiO sensitized with SQ2 (vide infra), the process of dark NiO oxidation interferes with that of the dye itself with the consequent detachment of the SQ2 sensitizer (Figures ESI13 and ESI14). 9 Figure ESI12. Cyclic voltammograms of bare NiO and NiO electrodes sensitised with N719, Black Dye and P1 with a scan rate of 20 mV s-1 for all samples. Figure ESI13. Cyclic voltammograms at 20 mV s-1 of (brown trace) bare NiO and (cyan trace) SQ2-sensitized NiO. 10 Figure ESI14. (Left) Cyclic voltammograms at 20 mV s-1 of bare NiO and NiO electrodes sensitised with SQ2 after 150 cycles; (right) picture of NiO electrode stripped of SQ2 dye after electrochemical cycling. According to literature data that report the HOMO level for the various dye-sensitizers in the non-anchored state and the upper edge of bare NiO VB (Figure ESI9),[28,33,36,ESI6] the oxidation of the dye within the examined range of applied potential (-0.1 - 1.3 vs NHE) should occur in all cases except P1 that presents the higherst oxidation potential in the isolated state (Figure ESI9). It must be taken into account also that pH, electrolyte composition and nature of the electrode can alter considerably the energy levels of the frontier orbitals as well as the potential of oxidation of the dye-sensitizer in passing from the free to the anchored state on a semiconducting oxide.[32b,ESI7] Similar to bare NiO, the oxide electrodes sensitised with N719, BD and P1 display a linear increase of the current density with scan rate (Figure ESI15). 11 Figure ESI15. Linear scan rate dependence of the amplitude of anodic peak I (Figure ESI8) of NiO-sensitised electrodes after 2h dipping in dye solutions. Such a trend suggests that the two oxidation reactions of dye-sensitized NiO are still under the kinetic control of a process which occurs at the surface of the oxide. This indicates also that such a process is not kinetically dependent by mass transport phenomena. In absence of any dye-based oxidation (cases of BD, N719 and P1, Figure ESI12) we can conclude that the current originated by the electrochemical oxidation of sensitized NiO produces a situation in which the dye is neutral and NiO is in an oxidized state. This recalls the condition of sensitised nickel oxide in an actual p-DSC at open circuit when the holes photoinjected in NiO are localized in the oxide layer and the dye has recovered the neutral state because of its regeneration by the oxidized form of the redox shuttle. The absence of any dye-related redox peak in the potential range in which NiO gets oxidized (Figure ESI12), is a measure of the kinetic stability of the dye in the anchored state when holes are present in the oxide and reveals the slowness of the back-recombination process: h+(NiO) + dye → [e--h+](NiO) + (dye)+ 12 Equation 3 where h+(NiO) represents an hole in the valence band (VB) of NiO and [e--h+](NiO) the recombination holeelectron within the VB of NiO following the transfer of an electron from the dye to the VB of NiO. The different shapes and width of the voltammograms (Figure ESI12) are associated with the ability of the dye of rendering less favorable the oxidation process of sensitized NiO compared to that of the bare oxide. The variations of current intensity in passing from bare to sensitized NiO are not ascribable to the variability of the NiO film thickness (l). The latter has a range of variability of about 10% with respect to the total thickness (l = 2.5±0.3 µm, vide supra) whereas the extent of the current variations between the differently sensitized NiO and bare NiO are generally much larger than 10% (Figures ESI3 and ESI12), and vary in a different way depending on the nature of the anodic process, i.e. if it is the I or the II anodic peak (Figure ESI3). For these reasons, the observed behaviour can be reasonably ascribed to the lower surface concentration of the NiO sites prone to oxidation after the sensitization of NiO surface with the dye molecules. Irrespective of the dipping time, P1, N719 and BD induce lower current densities in the sensitized systems with respect to NiO alone (Figure ESI12). In order to check the possible participation of the solvent of sensitization in the redox processes occurring at NiO electrode surface we carried out the cyclic voltammetry of bare NiO previously dipped in the pure solvents of sensitization, i.e. EtOH and acetonitrile. In presence of the sole solvent of sensitization we adopted the same time and temperature conditions of sensitization and post sensitization (see Text, Experimental Section). We could not observe any substantial difference between the voltammograms of NiO dipped respectively in pure EtOH and pure acetonitrile. This combination of facts led us to exclude the participation of EtOH in the redox processes observed in Figure ESI6 when NiO electrodes were sensitized in ethanol-based tincture solutions. As previously outlined, SQ2-sensitized NiO, once subjected to different scans of potential within the region of NiO oxidation, revealed a different behaviour (Figure ESI13) in comparison to P1, BD and N719 (Figure ESI12). The first voltammogram of SQ2-NiO presents two oxidation peaks with irreversible features at around 0.6 and 0.75 V vs Ag/AgCl. These peaks are not associable to NiO-based redox processes (Figure ESI3, Eqs. 1 and 2), and have to be ascribed to the oxidation of anchored SQ2 as evinced from the comparison of this voltammogram with that of similar squaraines in solution.[30] Upon electrochemical 13 cycling of SQ2-NiO there is an evolution of the voltammogram with the gradual disappearance of the peaks of SQ2 and the progressive restoration of the profile typical of bare NiO (Figure ESI13). At the end of the electrochemical cycling, sample SQ2-NiO underwent the detachment of the organic sensitizer as verified by the bleaching of the electrode (Figure ESI14, right panel). In addition to that, a change of the colour of the electrolytic solution was also observed at the end of the experiment with the electrolyte turning from pale yellow (original colour) to light blu, i.e. the characteristic colour of SQ2 dye. This combination of facts lead us to conclude that the oxidation of SQ2 anchored on NiO brought about its removal from nickel oxide surface. At this juncture, it should be outlined that repeated cycling of the p-DSC with SQ2 sensitizer under the conditions of Figures 6 (see Text) and ESI16 (vide infra) does not induce any substantial modification of the diode curve and tells us that the cathodic process of triiodide reduction (occurring at E < VOC) at the SQ2sensitized NiO electrode is not accompanied by any detachment of this sensitizer in presence of the redox couple I3-/ I- in the electrolyte. We checked the electrochemical stability of the SQ2-sensitized NiO electrode when the corresponding p-DSC is polarized in forward bias (Figure ESI16). The continuity of the dark JV curve of the SQ2 based p-DSC and the absence of new current peaks in passing from the potential range Eappl < VOC (see Text, Figure 6), to the range Eappl > VOC (Figure ESI16) is indicative of redox processes exclusively based on the I3-/ I- couple with I3- + 2 e- → 3 I- occurring at Eappl < VOC (see Text, Figure 6) and the reverse recombination process 3 I - → I3- + 2 e- taking place when VOC < Eappl < 0.04 V (Figure ESI16). Time-integration of the anodic currents in the voltammograms of the samples of Figure ESI12 gives the list of anodic charge values reported in Table ESI1. The integration of the anodic current associated with bare NiO gave the highest value of 5.4 mC cm-2, followed by N719, Black Dye and P1, respectively. Sensitization with P1 provoked the lowest value of exchanged charge density (3.1 mC cm-2). 14 Electrode Time-integration of the anodic currents (mC/cm2) Bare NiO 5.4 N719 Sensitised NiO 4.2 BD Sensitised NiO 3.6 P1 Sensitised NiO 3.1 Table ESI1. Time-integration of the anodic currents of all NiO electrodes here considered (data extracted from Figure ESI12). The general decrease of the anodic charge exchanged by NiO in passing from bare to sensitized can be considered as the equivalent of an effect of passivation of the NiO surface against its oxidation, which is introduced by the presence of the sensitizer. In other words, the number of the active sites of oxidation on the surface of NiO diminishes with sensitization. Such an effect of the chemisorbed dye is observable when NiO is brought to the potential of first oxidation.[4c] From the experiments of dye adsorption/desorption conducted on sensitized NiO utilizing 0.1M NaOH in EtOH as desorbing solution for the quantitative removal of the sensitizer, we found the following values of dye-loading (expressed as moles of dye per unit volume of NiO given in cubic centimetre): N719 (1.2x10-3); BD (0.9x10-3); SQ2 (3.2x10-4); P1 (1.7x10-4) (see Text, Table 1). The measured values of dye-loading show that there is no direct correlation between the amount of anchored dye and the extent of the decrease of the current peaks originated by NiO oxidation when different sensitizers are compared (Figure ESI12). This is because the amount of anodic charge exchanged by sensitized NiO (Table ESI1) is the lowest when P1 dye is used, i.e. the dye which is anchored at the smallest surface concentration (see Text, Table 1). Therefore, the decrease of the charge exchanged during NiO oxidation when NiO is in a sensitized state depends mainly on the nature of the anchored sensitizer rather than its surface concentration when different dyes have to be compared. The affirmation that the extent of the decrease of the current density for NiO oxidation is proportional directly to the percentage of the dye 15 coverage of the NiO surface retains its validity only if one refers exclusively to the same dye anchored at different surface concentrations and not to different dyes. In conclusion, we have found that dye sensitisation of NiO with N719, BD and P1 does not alter the nature of the redox processes of NiO in the examined range of potential due to the invariability of the potential at which NiO is oxidized when passing from the bare to the sensitised state (Figure ESI12). With the sole exception of NiO sensitization with SQ2 (Figure ESI13), the presence of the dye-sensitizer generally does not introduce additional redox peaks associated with the electroactivity of the dye-sensitizer (Figure ESI13).[4a,c,35] Under these circumstances the redox-inactive dyes, e.g. N719, BD and P1, form an apparently insulating layer that prevents the oxidation of NiO and the simultaneous ion intercalation in the oxide (Eqs. 1 and 2) in dark conditions.[4c] Dark characteristics of p-DSCs polarized in forward bias When p-DSCs are polarized in dark forward bias, i.e. Eappl ≥ VOC(dark) with Vinitial = VOC(dark), the profiles of the corresponding current appear as displayed in Figure ESI16. If the boundary conditions are modified (Vinitial = 0 V and Eappl ≥ 0), the dark current profiles change as shown in Figure ESI17. The p-DSCs employed in the experiments of Figures ESI16 and ESI17 are the same. 16 Figure ESI16. Dark current profile of the four DSCs with N719, SQ2, P1 and BD as sensitizers when the pDSCs are polarized in forward bias (Vinitial = VOC and Eappl ≥ VOC). For sake of comparison the dark current profile of the DSC with bare NiO as photocathode is also shown (black curve). In these conditions the observed current is generated by the anodic process of iodide oxidation. Figure ESI17. Dark current measurements of the four DSCs sensitised using N719, SQ2, P1 and BD sensitizers [Vinitial = 0 V (short circuit condition) and Eappl ≥ 0 V]. The observed anodic current originates from the same redox process producing the profiles of Figure ESI16. The dark current in the forward bias mode consists of the process of oxidative charge recombination 3I- → I3- + 2e- .[41] Such a process can be localized either at the NiO layer/electrolyte interface with possible mediation of the dye-sensitizer,[ESI9] or eventually at the TCO substrate/electrolyte interface.[ESI10] Dark 17 recombination in forward bias mode is somehow related to the overall performance of the p-DSCs under illumination[ESI11] although the sensitized semiconductor/electrolyte interface might undergo drastic changes in passing from illuminated to the dark condition.[ESI7a] In the experimental conditions of Figure ESI17 the potential at which there is the onset of oxidative charge recombination is higher for the most efficient p-DSCs that use P1 and SQ2 as sensitizers. In this respect there is coherence between the trends of the dark parameter of potential onset in Figure ESI17 and the parameter of JSC under illumination (Table 2) since the larger the onset of dark potential for the occurrence of charge recombination the larger the short circuit current in the illuminated p-DSCs. For this reason, the increase of the dark potential for the onset of charge recombination is a feature which must be sought after in order to improve the PV properties of the p-DSCs when NiO is the electrode material. Such a criterion holds when dark electrochemical properties are determined in the experimental conditions of Figure ESI17, i.e. Vinitial = 0 V (initial condition of short circuit) and Eappl ≥ 0 V. Figures ESI16 and ESI17 show analogous trends of growth rate for the anodic currents of recombination once the potential onset is surpassed with P1 and N719 sensitizers displaying the largest variations of dark recombination currents. In Figure ESI16 dark VOC represents the lower limit of potential beyond which there is the activation of the oxidation process 3 I - → I3- + 2 e- occurring with the eventual mediation of oxidized NiO (Figure ESI6). In the forward bias mode, i.e. for Eappl > VOC(dark), the recombination process: 3 I - + 2h+(NiO)→ I3- + 2 [h+-e-](NiO) Equation 4 takes place with the anodic current generated by the electrochemical injection of holes in NiO (see Eq.1 without considering the uptake of perchlorate anions) and the successive oxidation of iodide to triiodide (Eq.4). With the exception of BD, all the other dye-sensitizers act as electro-catalysts towards the recombination consisting in the oxidation of iodide with respect to bare NiO in dark conditions because of the decrease of the potential onset of iodide oxidation (i.e dark VOC) in comparison to bare NiO (Figure ESI16), (see also Figure 3 and Table 3 in Text). The largest rates of increase for the recombination current 18 according to Eq.4, are those induced by the sensitizers P1 and N719 when the two-electrode cell is polarized at Eappl > VOC(dark) (Figure ESI16). P1, N719 and SQ2 in the anchored state suggest the induction of a considerable dark electro-catalytic effect with respect to bare NiO. With the exception of SQ2 (Figure ESI13), both P1 and N719 proved to act as passivating agents towards the dark oxidation of NiO in redoxfree electrolyte (Figure ESI12). In other words, P1 and N719 impede the dark electrochemical injection of holes in NiO (Eqs.1 and 2), and the electro-kinetic effect they display in presence of redox shuttle with respect to bare NiO on the dark recombination process of Eq.4 can be associated reasonably to the favouring of the coupling between holes and I- once the holes have been electrochemically injected in NiO. The dyes P1, SQ2 and N719 immobilized on NiO act apparently as mediators in the dark recombination process of Eq.4. The anchoring of the dye does not modify the actual porosity of mesoporous NiO in passing from the bare to the sensitized state,[20] and the shifts of the potential onsets as well as the variations of recombination current intensities have to be ascribed mainly to the chemical nature of the dye and not to a possible modification of the morphology/surface area of the underlying oxide following its sensitization.[20,30] The possible participation of the FTO exposed to the electrolyte in the process of dark iodide oxidation of Figure ESI16 seems to be quite irrelevant from the analysis of the potential at which Ioxidizes on uncovered FTO (Figure ESI7). BD is the only dye that shifts the onset of the process of dark iodide oxidation at larger potential values with respect to bare NiO in both two-electrode (Figure ESI16) and (more slightly) in the three-electrode configuration (Figure ESI18). This indicates that the presence of BD prevents the dark oxidation of NiO (see voltammogram in Figure ESI12 determined in absence of redox shuttle) and its consequent activation for the successive oxidation of I- (Eq.4) with respect to the bare oxide. Therefore, BD behaves as a passivating agent in both processes of Eqs. 1,2 and 4, and excludes its participation as mediator in both dark oxidation of NiO and dark iodide oxidative recombination. 19 Figure ESI18. Dark voltammogram of NiO in the bare and BD sensitized states with the redox couple I-/I3-. Scan rate: 5 mV s-1. Electrolyte composition was 0.2 M I-/0.02 M I2 in MPN. Cell configuration was a threeelectrode cell with Ag/AgCl as reference electrode, and Pt as counter electrode. The two samples of RDS NiO have been sensitized with different duration of dipping time (1 hour, 1h, and overnight, ON, coloration). Dark parameters and photovoltaic performance of NiO based p-DSCs: some correlations In dark conditions the RDS NiO sample sensitized with P1 presented the voltammogram with the lowest current density in comparison to bare NiO and BD-, N719-sensitized oxide samples when the oxidation processes of Eqs. 1 and 2 were taking place and no redox shuttle was present in the electrolyte (Figure ESI12). This is equivalent to say that the dark electrochemical injection of holes in NiO (Eqs. 1 and 2) is rendered much more difficult by the presence of anchored P1 with respect to the other sensitizers when no redox-active couple is present in the electrolyte. When the redox couple is present in the electrolyte, in correspondence of the dark hole injection in bare NiO the consequent oxidation of iodide generates most of the current measured as determined by the comparison of the voltammograms in Figure ESI6. The current associated with the process of NiO oxidation (Eqs. 1 and 2) is much lower than the current produced by the oxidative recombination of iodide with the holes according to Eq. 4 (Figure ESI6). 20 When NiO sensitization with P1 is considered, the process of dark recombination between iodide and the holes injected electrochemically in NiO (see Eq. 4) gets kinetically favoured by the presence of the dye itself as observed by the steeper increase of the dark oxidation current (Figure ESI16 and ESI17) and the lower potential onset of iodide oxidation (Figure ESI16) in P1-sensitized NiO with respect to bare NiO. This behaviour under dark conditions is also consistent with the low recombination resistance in the EIS spectrum of P1-sensitized DSC under illumination (Figure 8). The observation of the largest conversion efficiency in P1-sensitized cell (Table 2) might appear a datum in contrast with the observation of good recombination properties of the P1-sensitized NiO electrode as determined by the experiments in dark conditions and under illumination. Actually the lowest Rr presented by the P1-sensitised p-DSC under illumination must be considered a consequence of the efficient hole injection in NiO effectuated by P1. The latter sensitizer, upon absorption of visible light (Figure ESI11), injects in NiO a much larger number of holes with respect to the other sensitizers here examined. This relatively large surface concentration of holes facilitates the reaction of recombination with iodide since the latter process is a bimolecular reaction the rate of which is directly proportional to the product between the iodide concentration and hole concentration at the electrode/electrolyte interface. Therefore, the increase of the rate of recombination has to be considered as originated by the large surface concentration of holes which gives rise to the appearance of a semicircle of recombination with the lowest resistance in the EIS spectrum with respect to the less efficacious sensitizers (Figure 8) In comparison to the other sensitizers, BD appears to be the dye with the most strong and complete antirecombination action. This is manifested through the low oxidation currents in the dark current profiles of Figures ESI16, ESI17, high potential onset of dark oxidative recombination (Figure ESI16) and the high recombination resistance in the EIS spectrum of the corresponding p-DSC under illumination (see Text, Figure 8). On the other hand the poor JV performance of the p-DSC sensitized with BD (see Text, Figure 2) has to be ascribed to an extremely ineffective process of photoinjection which is associated with low Rr due the quite low surface concentration of photoinjected holes. 21 References in Supplementary Information [ESI1] Marrani AG, Novelli V, Sheehan S, Dowling DP, Dini D (2014) Appl Mater Interfaces 6:143-152 [ESI2] A.G. Marrani, V. Novelli, M. Awais, D.P. Dowling, D. Dini, “Electrochemical and surface characterization of RDS NiO cathodes for dye-sensitized solar cells of p-type”, to be published [ESI3] Thorn RJ, Carlson KD, Crabtree GW, Wang HH (1985) J Phys C 18:5501-5510 [ESI4] Bard J, Faulkner LR (1980) Electrochemical Methods, Fundamental and Application. John Wiley & Sons, New York [ESI5] Chippindale AM, Dickens PG, Powell AV (1991) Progr Solid State Chem 21:133-198 [ESI6] (a) Wang ZS, Yamaguchi T, Sugihara H, Arakawa H (2005) Langmuir 21:4272-4276; (b) Geiger T, Kuster S, Yum JH, Moon SJ, Nazeeruddin MK, Gratzel M, Nuesch F (2009) Adv Funct Mater 19:2720-2727; (c) Daeneke T, Kwon TH, Holmes AB, Duffy NW, Bach U, Spiccia L (2011) Nature Chemistry 3:211-215 [ESI7] (a) Gregg BA (2004) Coord Chem Rev 248:1215-1224; (b) Zaban A, Ferrere S, Gregg BA (1998) J Phys Chem B 102:452-460 [ESI8] Clifford JN, Martınez-Ferrero E, Palomares E (2012) J Mater Chem 22:12415-12422 [ESI9] Ito S, Liska P, Comte P, Charvet R, Pachy P, Bach U, Schmidt-Mende L, Zakeeruddin SM, Kay A, Nazeeruddin MK (2005) Chem Commun:4351-4353 [ESI10] Menzies DB, Dai Q, Cheng YB, Simon GP, Spiccia L (2006) Compt Rend Chimie 9:713-716 22