Sample
advertisement

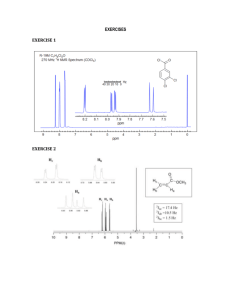
Chem 4000 Final Examination Spring 2008 April 24 2008 Part I Do all two sections (20 marks total) Section A Answer two of the following question in a few sentences (5 marks each) 1) Name the following spin systems: i) C13HF3 iii) ii) CH3CH2OH iv) CH3CH2CHClCH3 H Br H H H H Br H 2) Draw the Karplus curve and give a physical justification for this behavior, and explain how it is applied.. 3) Explain why the chemical shift of an aldehyde proton is large while that of a terminal alkyne is small. Section B Answer two of the following question in a few sentences (5 marks each) 1) Give four reasons why 13C NMR is much less sensitive than 1H NMR. 2) If a carbon has a spin-spin relaxation time of 3.2 seconds and a spin lattice relaxation time of 10.5 seconds, determine the optimum relaxation delay and acquisition time that ensures quantitative results and avoids clipping the FID. 3) Explain why the HMBC and HSQC sequences are referred to as inverse detected experiments, in your own words explain how they differ with, and what advantages they have over direct methods. Part II Determine the structure of one of the following three systems given on the following three pages. Justify your answer using: i) Determine empirical formula and determine the DBE. ii) Give the MS fragmentation pattern indicating the diagnostic fragments. iii) Account for the 1H NMR coupling patterns. iv) Account for the 1H chemical shifts. v) Compute the 13C chemical shifts. vi) Account for phases observed in the DEPT 90 and 135 spectra. (total of 30 marks) Question 1 Question 2 Question 3 Part III Determine the structure based on the spectra given one the following two pages. Justify your answer using: i) Determine empirical formula and determine the DBE. ii) Give the MS fragmentation pattern accounting for the diagnostic fragments. iii) Account for the 1H NMR coupling patterns. iv) Account for the 1H chemical shifts. v) Compute the 13C chemical shifts. vi) Account for phases observed in the DEPT 90 and 135 spectra. vii) Account for the correlations observed in the COSY spectrum. viii) Account for the correlations observed in the HSQC spectrum. (total of 40 marks) Question 1 Part V Do one of the following two questions. (total of 40 marks) Question 1 a) Sketch the energy level diagram for an ABC system, indicate all the allowed transitions, and predict how many lines are present in the spectrum. b) Sketch the energy level diagram for an ABX system, indicate all the allowed transitions, sketch the spectrum and predict how many lines are present in the spectrum. c) Sketch the energy level diagram for an AMX system, indicate all the allowed transitions, sketch the spectrum and predict how many lines are present in the spectrum. d) Sketch the energy level diagram for an A2X system, indicate all the allowed transitions, sketch the spectrum and predict how many lines are present in the spectrum. Question 2 Determine the structure based on the spectra given one the following two pages. Justify your answer using: i) Determine empirical formula and determine the DBE. ii) Give the MS fragmentation pattern accounting for the diagnostic fragments. iii) Account for the 1H NMR coupling patterns. iv) Account for the 1H chemical shifts. v) Compute the 13C chemical shifts. vi) Account for phases observed in the DEPT 90 and 135 spectra. vii) Account for the correlations observed in the COSY spectrum. viii) Account for the correlations observed in the HSQC spectrum.