Appendix S2: Specimens used in this study
advertisement
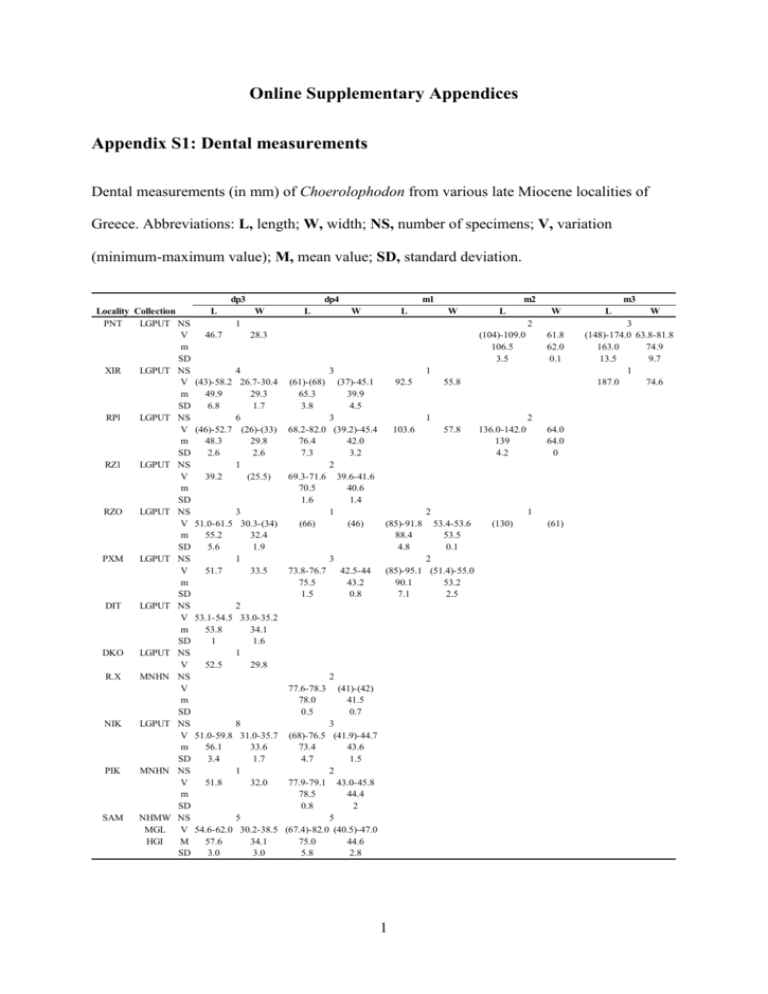
Online Supplementary Appendices Appendix S1: Dental measurements Dental measurements (in mm) of Choerolophodon from various late Miocene localities of Greece. Abbreviations: L, length; W, width; NS, number of specimens; V, variation (minimum-maximum value); M, mean value; SD, standard deviation. dp3 Locality Collection PNT LGPUT NS V m SD XIR LGPUT NS V m SD RPl LGPUT NS V m SD RZ1 LGPUT NS V m SD RZO LGPUT NS V m SD PXM LGPUT NS V m SD DIT LGPUT NS V m SD DKO LGPUT NS V R.X MNHN NS V m SD NIK LGPUT NS V m SD PIK MNHN NS V m SD SAM NHMW NS MGL V HGI M SD L dp4 W L m1 W L m2 W L 1 46.7 m3 W 2 28.3 4 (43)-58.2 26.7-30.4 49.9 29.3 6.8 1.7 6 (46)-52.7 (26)-(33) 48.3 29.8 2.6 2.6 1 39.2 (25.5) 3 51.0-61.5 30.3-(34) 55.2 32.4 5.6 1.9 1 51.7 33.5 (104)-109.0 106.5 3.5 3 (61)-(68) 65.3 3.8 1 (37)-45.1 39.9 4.5 92.5 3 68.2-82.0 (39.2)-45.4 103.6 76.4 42.0 7.3 3.2 2 69.3-71.6 39.6-41.6 70.5 40.6 1.6 1.4 1 (66) (46) (85)-91.8 88.4 4.8 3 73.8-76.7 42.5-44 (85)-95.1 75.5 43.2 90.1 1.5 0.8 7.1 2 53.1-54.5 33.0-35.2 53.8 34.1 1 1.6 1 52.5 29.8 2 77.6-78.3 78.0 0.5 61.8 62.0 0.1 (41)-(42) 41.5 0.7 8 51.0-59.8 31.0-35.7 56.1 33.6 3.4 1.7 1 51.8 32.0 3 (68)-76.5 (41.9)-44.7 73.4 43.6 4.7 1.5 2 77.9-79.1 43.0-45.8 78.5 44.4 0.8 2 5 5 54.6-62.0 30.2-38.5 (67.4)-82.0 (40.5)-47.0 57.6 34.1 75.0 44.6 3.0 3.0 5.8 2.8 1 55.8 1 2 57.8 136.0-142.0 139 4.2 53.4-53.6 53.5 0.1 (130) 2 64.0 64.0 0 1 2 (51.4)-55.0 53.2 2.5 (61) L W 3 (148)-174.0 63.8-81.8 163.0 74.9 13.5 9.7 1 187.0 74.6 L 26.6-27.3 27.0 0.3 26.4-28.7 27.6 1.6 29.0-30.8 29.8 0.9 (25)-27.0 26.0 1.4 29.0-34.5 32.0 2.3 33.0 32.0 W 4 19.5-22.9 21.3 1.8 2 (19.8)-23.2 21.5 2.4 3 20.6-23 21.8 1.2 2 20.7-21.6 21.2 0.6 4 24.1-27.0 25.3 1.3 1 23.0 1 24.4 7 33.0-36.0 22.2-27.8 24.0 34.3 2.0 1.1 1 26.5 34.5 7 30.6-35.3 22.0-27.0 24.6 33.2 1.9 1.6 1 23.2 34.6 L W L M2 M1 DP4 DP3 DP2 Locality Collection LGPUT NS PNT V M SD LGPUT NS XIR V M SD LGPUT NS RPl V M SD LGPUT NS RZ1 V M SD LGPUT NS RZO V M SD LGPUT NS VTK V LGPUT NS DIT V MNHN NS R.X V LGPUT NS NIK V M SD MNHN NS PIK V M SD NHMW NS SAM V MGL M HGI SD MTLB ΝΗΜΑ NS V W 3 41.2-46.7 33.0-38.2 (65.4)-70.0 (47.3)-50.5 48.4 67.4 35.5 44.1 1.8 2.4 2.0 1.9 5 7 46.5-50.4 (37.1)-42.8 67.6-78.5 (37)-53.7 47.1 71.4 41 48 6.8 4.2 2.4 1.5 1 (51) 73.6 W L W L 7 1 81.7 (78.2) 56.3 2 (53.2)-(54.4) 53.8 0.8 1 (45) 2 2 80.0 41.5-42.7 61.5 (40)-40.3 35.0-(35.8) 42.1 35.4 40.2 0.8 0.6 0.2 2 6 46.5-53.1 40.6-46.6 72.5-78.7 (53.7)-55.2 54.5 75.6 43.9 49.3 1.1 4.4 2.2 2.2 1 1 51.5 (76) 46.7 57.2 1 1 (39) 76.6 42.5 52.6 1 48.6 72.5 1 8 (47) (67) (45)-56.5 41.5-45.0 43.3 51.5 1.5 3.4 2 2 49.5-52.7 44.9-45.0 75.3-77.0 (48)-50.2 49.1 76.2 45 51.1 1.6 1.2 0.1 2.3 2 6 6 60.1-60.4 47.6-54.0 39.0-45.5 (65)-73.0 (47)-53.0 89.4-94.1 60.3 91.8 50.1 69 42.8 51.1 0.2 3.3 2.3 3.0 2.8 2.7 1 (68) (87) 2 1 69.0 107.0 1 (126) (62) Appendix S2: Specimens used in this study Choerolophodon anatolicus Pentalophos-1 (PNT, Axios Valley, early Vallesian, LGPUT): Maxillary fragment with DP2DP3 dex, PNT-159; 2 maxillary fragments with DP2-DP4 sin, PNT-94, 157; DP2 dex, PNT45; DP3 dex, PNT-46; 3 DP3 sin, PNT-16a, 47, 93; DP4 sin, PNT-16b; dp3 sin, PNT-158; right mandibular fragment with m2 and erupting m3, PNT-154; left mandibular fragment with m2 and mesial part of erupting m3, PNT-153; m3 dex, PNT-155; left hemimandible with m3, PNT-156. Choerolophodon pentelici Xirochori-1 (XIR, Axios Valley, late Vallesian, LGPUT): I2 of juvenile individual, XIR-18; DP2 dex, XIR-10; 2 DP3 dex, XIR-12, 13; DP3 sin, XIR-11; DP4 dex, XIR-14; maxillary fragment with DP4 sin, XIR-7; maxilla with DP2 dex and DP3-DP4 dex and sin, XIR-21; cranium with I2, DP3-DP4 dex and sin, XIR-23; M1 dex, XIR-15; right mandibular fragment with dp3, XIR-16; left mandibular fragment with dp3, XIR-17; mandible with dp3-dp4 dex and sin, XIR-20; left mandibular fragment with partial dp4 and m1 in alveolus, XIR-22; left mandibular fragment with dp4-m1 and m2 in alveolus, XIR-9; m3 sin, XIR-6. Ravin de la Pluie (RPl, Axios Valley, late Vallesian, LGPUT): 2 DP2 dex, RPl-65, 253; DP2 sin, RPl-254; part of DP3 sin, RPl-265; DP4 dex, RPl-21; M1 sin, RPl-24; M1-M2 dex, RPl23; 4 dp3 dex, RPl-22, 259, 260, 266; left mandibular fragment with dp3, RPl-258; 2 left mandibular fragment with dp3-dp4, RPl-256,257; left mandible with dp3-m1, RPl-255; left mandibular fragment with m2 in alveolus, RPl-275. 3 Ravin des Zouaves-1 (RZ1, Axios Valley, late Vallesian, LGPUT): cranium with I2, DP2DP4 and Μ1 in alveolus dex and sin, and associated mandible with dp3-dp4 and m1 in alveolus dex and sin, RZ1-9. Ravin des Zouaves-5 (RZO, Axios Valley, early Turolian, LGPUT): 3 I2 of juvenile individuals, RZO-220, 221, 238; DP2 dex, RZO-219; DP2 sin, RZO-218; cranium with I2, DP2-DP4 dex and sin, and associated mandible with dp3 and dp4 in alveolus dex and sin, RZO-208; 2 DP3 dex, RZO-12, 207; DP3 sin, RZO-13; cranium fragment with DP3-DP4 dex, RZO-11; dp3 sin, RZO-192; mandible with dp4 sin, m1 dex and sin, m2 dex in alveolus, RZO-206. Vathylakkos-2 (VTK, Axios Valley, middle Turolian, LGPUT): left maxillary fragment with DP2-DP3 (broken), VTK-90; left maxillary fragment with DP3-DP4, VTK-41. Prochoma-1 (PXM, Axios Valley, middle Turolian, LGPUT): DP3, PXM-173; dp3 dex, PXM-262; dp4 dex, PXM-261; right and left mandibular frag. with dp4-m1, PXM-176. Dytiko-2 (DIT, Axios Valley, late Turolian, LGPUT): 2 I2 of juvenile individuals, DIT-30, 31; right cranium fragment with I2 and the juvenile dentition inside the bone, DIT-26; partial cranium with I2 sin, DP2 dex and sin and DP3-DP4 in alveolus dex and sin, DIT-32; right mandibular fragment with dp3, DIT-28; left mandibular fragment with dp3, DIT-29; right mandibular fragment with dp3 and dp4 in alveolus, DIT-27. Dytiko-3 (DKO, Axios Valley, late Turolian, LGPUT): left mandible with dp3, DKO-42. Ravin X (R.X, Axios Valley, Turolian): DP4 sin, MNHN-SLQ-3; mandible with dp4 dex and sin, MNHN-SLQ-2. Nikiti-2 (ΝΙΚ, Chalkidiki, early Turolian, LGPUT): I2 dex of adult individual, ΝΙΚ-1776; 4 I2 of juvenile individuals, NIK-1016, 1606, 1607, 1614; 3 DP2 dex, NIK-1591; 5 DP2 sin, NIK-184, 1608, 1609, 1610, 1628; 2 DP3 dex, NIK-849, 1605; 2 DP3 sin, NIK-261, 1611; left maxillary fragment with DP2-DP3, NIK-1589; maxilla with DP3-DP4 dex and DP2-DP4 sin, ΝΙΚ-1615; maxillary fragment with DP3 and DP4 sin in alveolus, NIK-1613; 2 dp3 dex, 4 NIK-1586, 1590; dp4 dex, NIK-1612; dp4 sin, NIK-262; right and left hemimandibles with dp3, NIK-1592; dp3 dex and left hemimandible with dp3, NIK-1604; 2 left mandibular fragments with dp3 in alveolus, ΝΙΚ-1585, 1588; left mandibular fragment with dp4 in alveolus, ΝΙΚ-1587; mandible with dp3-dp4 and m1 in alveolus dex και sin, NIK-1593. Samos (SAM, Samos Island, Turolian): cranium with DP2-DP3 and DP4 in alveolus dex and sin, and associated mandible with dp3 dex and sin, NHMW 2014/0129/0002; cranium with I2, DP2-DP4 dex and sin, and associated mandible with dp3 and erupting dp4 dex and sin, NHMW 2014/0129/0001; cranium with I2, DP2-DP4 dex and sin, and associated left mandible with dp3-dp4 and m1 in alveolus, HGI-Ok-548, 549; cranium with I2, DP4-M1 dex and sin, NHMW 1913/0001/0017; DP2 dex, MGL-S 614; right and left mandibular fragment with dp3, MGL-S 340; right mandibular fragment with dp4 and left mandibular fragment with dp4 and partial m1, MGL-S 333. Mytilinii-1B (MTLB, Samos Island, middle Turolian, NHMA): cranium fragment with M1M2 dex and M2 sin, MTLB-126; DP2 sin, MTLB-11. Pikermi (Attica, middle Turolian, MN 12): juvenile cranium with DP2-DP4 dex, I2 sin and DP4 sin, and associated mandible with dp3-dp4 dex and dp4 sin, MNHN-PIK-3665; DP3 sin, MNHN-PIK-1705. 5 Appendix S3: Character-taxon matrix used for the phylogenetic analyses hypothetical ancestor C. kisumuensis C. palaeindicus C. zaltaniensis C. ngorora- 1 C. guangheensis C. chioticus C. anatolicus C. pentelici C. corrugatus C. ngorora- 2 1 0 1 1 1 1 1 1 1 1 1 1 2 0 0 0 1 1 0 0 2 2 2 2 3 0 0 0 1 1 0 0 2 2 2 2 4 0 0 ? 1 1 0 0 2 2 2 2 5a 0 1 ? ? 1 1 1 1 1 1 1 6 0 0 0 0 1 ? ? 1 1 1 ? 7b 0 0 0 0 0 ? ? 0 1 1 ? 8 0 0 ? 1 1 1 1 1 1 1 1 9 0 ? ? 0 0 ? ? 0 1 1 ? 10 0 ? ? ? 0 ? ? 0 1 1 ? 6 11 0 ? ? ? 0 ? ? 0 1 0 ? 12 0 ? ? ? ? ? ? 0 1 1 ? c d e f 13 14 15 16 17 18 19 0 0 0 0 0 0 0 ? ? ? ? ? 0 0 ? ? ? ? ? ? ? ? ? ? 0 ? ? ? ? 1 ? ? ? ? ? ? ? ? ? ? 1 0 ? 1 ? 0 ? 0 0 0 1 0 1 1 1 1 1 0 0 1 1 1 1 0 1 1 1 0 0 1 ? ? ? ? ? ? ? 20 0 0 ? ? ? 0 0 1 1 1 ? 21 0 0 ? ? ? 0 0 1 2 1 ? 22 0 0 ? ? ? 0 0 0 1 0 ? 1. Intermediate molars without chevroning (0) or with chevroning (1) 2. Teeth with absent/weak ptychodonty (0), intermediate (1) or strong ptychodonty (2) 3. Teeth with absent/weak choerodonty (0), intermediate (1) or strong choerodonty (2) 4. Teeth with absent/weak cementodonty (0), intermediate (1) or strong cementodonty (2) 5. Upper tusks with enamel band (0) or without (1) 6. m3 with four lophids (0) or may have five lophids (1) 7. In tetralophodont m3 the distal cingulum may be connected with the fourth lophid (0) or not (1) 8. M3 with three lophs (0) or more (1) 9. The size of DP2/dp3/DP3 is small (0) or large (1) 10. The development of the distal cingulum in dp3 is small (0) or large (1) 11. The distal cingulum in dp3 is connected with the second lophid (0) or isolated (1) 12. The development of the distal cingulum in DP3 is small (0) or large (1) 13. In DP3 the metacone is connected with the posttrite conelet of the distal cingulum (0) or separated (1) 14. The mandibular symphysis in adult individuals is ventrally not deflected (0) or deflected (1) 15. The mandibular symphysis in juvenile individuals is ventrally not deflected (0) or deflected (1) 16. Corpus and ramus are almost vertical (0) or form obtuse angle (1) 7 17. Retromolar gap is not present (0) or present (1) 18. Steeply inclined facial region/upper tusk emerge downwards (0) or moderately inclined facial region/upper tusk emerge sub-horizontally (1) 19. Narrow parietal region (0) or wide parietal region (1) 20. Orbit set low (0) or at the top of the cranium (1) 21. Orbit located above the last molar in function (0), just behind (1) or remote (2) 22. The anterior zygomatic process is located at the level of the last molar in function (0) or behind (1) a Palaeomastodon and Phiomia from the Oligocene of Africa, as well as the primitive elephantoids from the early Miocene (e.g. Eozygodon morotoensis, Gomphotherium sylvaticum, Archaeobelodon filholi) presented a lateral enamel band (Tassy 1977, 1986, 1996; Pickford & Tassy 1980; Sanders et al. 2010), whereas more advanced elephantoids, such as Mammut and Tetralophodon, lack enamel band (Göhlich 1999). b In the primitive gomphotheres of the Gomphotherium “annectens group” (G. annectens, G. cooperi, G. sylvaticum, G. sp.) from the early Miocene of the Old World, the m3 has four lophids without isolated distal cingulum (Tassy 1977, 1985, 1996). Therefore the connection between fourth lophid and distal cingulum is a plesiomorphic character, which in choerolophodonts in present until C. anatolicus. c Tassy (1985, 1986) mentioned that the symphysis in Gomphotherium annectens and Archaeobelodon filholi is located at the extension of the corpus and considered the absence of deflection a plesiomorphic character. 8 d In G. angustidens and G. subtapiroideum the symphysis in juvenile mandibles is straight (Tassy 1985, 1994; Göhlich 2010). e Phiomia, Gomphotherium subtapiroideum, G. steinheimense and G. angustidens form almost vertical angle, whereas in the more primitive Numidotherium and Moeritherium the angle is acute (Tassy 1985; Göhlich 1998, 2010; Sanders et al. 2010). f In the primitive elephantoids from the early Miocene of Africa, such as Archaeobelodon, the facial region is inclined and the tusks emerge downwards (Tassy 1986: pl. 3). 9 Appendix S4: Methodology used for dental microwear analysis According to Kay & Hiiemae (1974) the masticatory process of the food is divided into the shearing phase with parallel movements of the jaw to the occlusal surfaces, and the grinding phase with additional vertical movements. Upper and lower teeth were not separated (Teaford & Walker 1984). For the mold acquisition we followed the protocol of Merceron et al. (2012). The appropriate specimens were selected and cleaned with acetone in order all traces of glue from the preparation to be removed. For each selected surface, casts were produced using polyvinyl siloxane (Regular Body Microsystem, Coltene President). Each cast was placed horizontally in a framework (Putty soft, Coltene President), which was subsequently poured with transparent polyurethane resin (Ebalta MG709/20) and placed in an autoclave with temperature of about 50° C and pressure of 2 × 105 Pa for 5 hours. With this procedure the casts are without bubbles either on their surface or inside. For the image analysis we followed the protocol of Merceron et al. (2004, 2005, 2012). The tooth surfaces were digitized (1μm = 1 pixel) to 256 Gy level with magnification ×30, using a CCD camera (with lenses ×0.5 Leica ICD, Leica Microsystems) connected to a light stereomicroscope (×60, Leica MZ 125, Leica Microsystems). Each photo was enlarged to ×120 for use on the computer screen. Analysis of images was conducted by ImageJ (Abràmoff et al. 2004) and the plug-in ObjectJ (Vischer et al. 1994). Then a 0.09mm2 square was defined, where the counting of all the microwear scars took place. Counted were all the scars that were inside this square or passed through it. The microwear scars that were recognized on the enamel were divided into the long scars (scratches) and the small round scars (pits). The distinction between scratches and pits was based on the ratio width/length. For scratches the ratio is less than 1/4, wheares for pits is 10 greater. Apart from this, a classification in small and large sizes was conducted, respectively. The length of the wide scratches and the length of the major axis of the large pits is greater than 15μm. Moreover the percentage of pits (Pp) according to the type Pp = 100 × Np / (Np + Ns) was calculated. The data obtained from image analysis were statistically processed using the software PAST version 2.14 (Hammer et al. 2001). Because the values of the variables do not follow a normal distribution, thus they do not fulfill the conditions for parametric tests, they were rank transformed before analysis. Two sets of one-way analysis of variance (ANOVA) were performed, one for comparison among juvenile and adult specimens of Choerolophodon and a second for comparison among adult specimens of C. anatolicus, C. pentelici, D. giganteum, G. subtapiroideum and G. steinheimense. The analysis of variance examines the null hypothesis (Ho - the specimens have a similar microwear pattern) at significance a = 0.05. When the hypothesis is rejected some of the species differ significantly. To identify precisely the pairs of species, post hoc pair-wise comparison was applied, using the Tukey’s Honestly Significant Difference (HSD), the most conservative one in investigating differences, providing the greatest safety for errors. The herbivorous animals can be divided into three main dietary categories depending on their dietary habits: grazers, whose main dietary source are graminoids (grasses, sedges, rushes) and exhibit higher density of scratches than pits; browsers, whose diet contain more soft food, such as leaves and fruits and exhibit fewer scratches; and, mixed feeders, which represent an intermediate category and the type of food intake depends on the environmental conditions of the time of death of the animal (Hofmann & Stewart 1972; Merceron et al. 2007). The three main variables used for the distinction are the number of scratches (Ns), the number of pits (Np) and the percentage of pits (Pp), the latter being the most important in distinguishing grazers from browsers. Low Pp (<40%) indicates that the predominant diet are 11 graminoids, whereas high Pp (>60%) indicates a relatively soft diet. Percentage ranging from 40% to 60% characterizes a mixed diet (Merceron et al. 2012). 12 Appendix S5: List of specimens used for dental microwear analysis Species Ontogenetic stage C. anatolicus adult C. pentelici C. anatolicus C. pentelici juvenile Locality Pentalophos-1 Biozone Specimen ΜΝ 9 PNT-153 PNT-154 PNT-155 PNT-156 Xirochori-1 ΜΝ 10 XIR-6 XIR-9 Ravin de la Pluie ΜΝ 10 RPl-23 RPl-24 Ravin des Zouaves-5 ΜΝ 11 RZO-206 Pentalophos-1 ΜΝ 9 PNT-94 Xirochori-1 ΜΝ 10 XIR-7 XIR-11 XIR-12 XIR-17 XIR-20 XIR-21 XIR-22 XIR-23 Ravin de la Pluie ΜΝ 10 RPl-256 RPl-257 RPl-259 Ravin des Zouaves-1 ΜΝ 10 RZ1-9 Ravin des Zouaves-5 ΜΝ 11 RZO-12 RZO-13 RZO-208 RZO-208 Prochoma-1 ΜΝ 12 PXM-176 PXM-261 Vathylakkos-2 ΜΝ 12 VTK-41 Dytiko-3 ΜΝ 13 DKO-42 Nikiti-2 ΜΝ 11 NIK-1592 NIK-1604b 13 Tooth Shearing facet Grinding facet m2 + m2 + + m3 + + m3 + + m3 + + m1 + + M1 + M1 + m1 + + DP3 + DP4 + DP3 + DP3 + dp3 + dp4 + DP3 + dp4 + DP3 + dp4 + dp4 + + dp3 + dp4 + DP3 + + DP3 + dp3 + dp4 + dp4 + + dp4 + + DP3 + + dp3 + + dp3 + dp3 + Appendix S6: Summary statistics of dental microwear variables A, juvenile and adult specimens of Choerolophodon; B, adult specimens of Choerolophodon, Deinotherium and Gomphotherium. Data of Deinotherium and Gomphotherium from Calandra et al. (2008). Abbreviations: N, number of individuals; Μ, mean value; SΕΜ, standard error of the mean; Ns, number of scratches; Ls, length of scratches; Np, number of pits; Nws, number of wide scratches; Nlp, number of large pits; Pp, percentage of pits. Facet N A adult adult juvenile juvenile B Choerolophodon anatolicus Choerolophodon pentelici Deinotherium giganteum Gomphotherium steinheimense Gonphotherium subtapiroideum shearing grinding shearing grinding 9 6 22 7 M 18,0 17,5 21,7 21,9 shearing grinding shearing grinding shearing grinding shearing grinding shearing grinding 4 3 5 3 17 17 13 11 6 5 19,7 15,7 16,6 19,3 16,3 22,7 26,6 19,4 30,2 31,2 Ns SEM ± 0,9 ± 2,0 ± 0,8 ± 2,7 M 308,3 318,3 213,9 205,4 Ls SEM ± 38,5 ± 27,6 ± 16,3 ± 54,2 M 4,8 6,5 5,3 6,2 Np SEM ± 0,6 ± 1,5 ± 0,3 ± 1,0 M 1,6 1,3 1,3 1,5 Nws SEM ± 0,3 ± 0,4 ± 0,1 ± 0,4 M 0,8 0,4 0,9 1,1 Nlp SEM ± 0,2 ± 0,2 ± 0,1 ± 0,3 M 20,7 27,4 19,7 22,2 Pp SEM ± 2,1 ± 6,3 ± 0,8 ± 2,6 ± 1,2 ± 2,5 ± 0,9 ± 3,2 ± 1,1 ± 2,5 ± 2,7 ± 2,6 ± 2,2 ± 3,0 302,0 358,9 313,4 277,7 319,4 358,6 299,6 252,5 300,0 191,9 ± 60 ± 36,3 ± 56,2 ± 29,0 ± 34,9 ± 30,5 ± 23,5 ± 24,4 ± 46,6 ± 23,8 4,9 ± 0,4 8,3 ± 2,7 4,7 ± 1,1 4,7 ± 0,9 73,6 ± 5,8 51,2 ± 5,0 69,8 ± 9,2 63,9 ± 11,5 69,9 ± 7,0 72,5 ± 10,1 2,1 1,0 1,3 1,5 0,4 0,6 0,4 0,7 1,3 0,6 ± 0,4 ± 0,3 ± 0,3 ± 0,8 ± 0,1 ± 0,1 ± 0,1 ± 0,2 ± 0,5 ± 0,2 0,8 0,8 0,7 0,0 7,1 5,3 3,9 3,2 3,0 3,5 ± 0,3 ± 0,2 ± 0,3 0 ± 0,7 ± 0,7 ± 0,8 ± 0,4 ± 0,9 ± 1,2 20,0 34,4 21,4 20,5 79,8 66,9 68,9 72,9 69,0 68,3 ± 0,9 ± 10,7 ± 3,9 ± 5,8 ± 3,0 ± 4,8 ± 4,7 ± 4,4 ± 3,6 ± 5,7 14 Appendix S7: Anova summary for juvenile and adult specimens of Choerolophodon The bold values indicate p values lower than 0.05. Abbreviations: f, degrees of freedom; SS, sums of squares; MS, mean square; F, F-value; p, probability. Ontogenetic stage Residuals Total df 1 29 30 Ontogenetic stage Residuals Total df 1 29 30 Ontogenetic stage Residuals Total df 1 29 30 Ontogenetic stage Residuals Total df 1 11 12 Ontogenetic stage Residuals Total df 1 11 12 Ontogenetic stage Residuals Total df 1 11 12 SHEARING Ns (ranked data) SS MS F p 524,93 524,93 8,25 0,008 1845,84 63,65 2370,77 Np (ranked data) SS MS F p 59,63 59,63 0,73 0,401 2379,72 82,06 2439,35 Nlp (ranked data) SS MS F p 13,33 13,33 0,17 0,686 2318,22 79,94 2331,55 GRINDING Ns (ranked data) SS MS F p 15,17 15,17 1,00 0,339 166,83 15,17 182,00 Np (ranked data) SS MS F p 0,05 0,05 0,00 0,958 172,26 15,66 172,31 Nlp (ranked data) SS MS F p 21,76 21,76 2,09 0,176 114,55 10,41 136,31 15 Ls (ranked data) SS MS F p 391,41 391,41 5,44 0,03 2088,59 72,02 2480,00 Nls (ranked data) SS MS F p 142,12 142,12 1,85 0,185 2232,59 76,99 2374,71 Pp (ranked data) SS MS F p 1,15 1,15 0,01 0,909 2501,82 86,27 2502,97 Ls (ranked data) SS MS F p 69,64 69,64 6,82 0,024 112,36 10,21 182,00 Nls (ranked data) SS MS F p 8,97 8,97 0,67 0,430 147,33 13,39 156,31 Pp (ranked data) SS MS F p 1,24 1,24 0,08 0,789 180,76 16,43 182,00 Appendix 8: Anova summary for various proboscidean species The bold values indicate p values lower than 0.05. Abbreviations: f, degrees of freedom; SS, sums of squares; MS, mean square; F, F-value; p, probability. df Species 4 Residuals 40 Total 44 df Species 4 Residuals 40 Total 44 df Species 4 Residuals 40 Total 44 df Species 4 Residuals 34 Total 38 df Species 4 Residuals 34 Total 38 df Species 4 Residuals 34 Total 38 SHEARING Ns (ranked data) Ls (ranked data) SS MS F p SS MS F p 3532,53 883,13 8,80 <0,001 63,74 15,94 0,08 0,987 4014,67 100,37 7526,26 188,16 7547,20 7590,00 Np (ranked data) Nws (ranked data) SS MS F p SS MS F p 3694,22 923,55 9,38 <0,001 2604,08 651,02 8,74 <0,001 3939,70 98,49 2979,12 74,48 7633,91 5583,20 Nlp (ranked data) Pp (ranked data) SS MS F p SS MS F p 3928,28 982,07 11,79 <0,001 4396,45 1099,11 13,77 <0,001 3332,70 83,32 3193,55 79,84 7260,98 7590,00 GRINDING Ns (ranked data) Ls (ranked data) SS MS F p SS MS F p 1121,51 280,38 2,53 0,006 1499,17 374,79 3,70 0,013 3774,80 111,02 3440,83 101,20 4896,31 4940,00 Np (ranked data) Nws (ranked data) SS MS F p SS MS F p 2227,30 556,83 7,00 <0,001 584,60 146,15 1,20 0,327 2704,60 79,55 4130,32 121,48 4931,90 4714,92 Nlp (ranked data) Pp (ranked data) SS MS F p SS MS F p 2059,77 514,94 7,03 <0,001 1654,89 413,72 4,28 0,007 2492,13 73,30 3285,11 96,62 4551,90 4940,00 16 Appendix S9: Post hoc HSD Test Post hoc HSD test among the adult specimens of the various proboscidean species. SHEARING C. anatolicus C. pentelici D. giganteum G. steinheimense G. subtapiroideum GRINDING C. anatolicus C. pentelici D. giganteum G. steinheimense G. subtapiroideum C. anatolicus C. pentelici D. giganteum G. steinheimense G. subtapiroideum Np, Nws, Nlp, Pp Np, Nws, Nlp, Pp Np, Nws, Pp Np, Nws, Pp Ns Np, Pp Ns, Np, Pp Ns, Nlp C. anatolicus C. pentelici D. giganteum G. steinheimense G. subtapiroideum Nlp Np, Pp Ns, Np Np, Nlp, Pp Np, Nlp, Pp Np, Nlp 17 References Abràmoff, M. D., Magalhães, P. J. & Ram, S. J. 2004. Image processing with ImageJ. Biophotonics International, 11, 36–42. Calandra, I., Göhlich, U. B. & Merceron, G. 2008. How could sympatric megaherbivores coexist? Example of niche partitioning within a proboscidean community from the Miocene of Europe. Naturwissenschaften, 95, 831–838. Göhlich, U. B. 1998. Elephantoidea (Proboscidea, Mammalia) aus dem Mittel- und Obermiozän der Oberen Süβwassermolasse Süddeutschlands: Odontologie und Osteologie. Münchner Geowissenschaftliche Abhandlungen, Reihe A, Geologie und Paläontologie, 36, 1–245. Göhlich, U. B. 1999. Order Proboscidea. Pp. 157–168 in G. E. Rössner and K. Heissig (eds) The Miocene land mammals of Europe. Verlag Dr. Friedrich Pfeil, München. Göhlich, U. B. 2010. The Proboscidea (Mammalia) from the Miocene of Sandelzhausen (southern Germany). Palaontologische Zeitschrift, 84, 163–204. Hammer, Ø., Harper, D. A. T. & Ryan, P. D. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 1–9. Hofmann, R. R. & Stewart, D. R. M. 1972. Grazer or browser: a classification based on the stomach structure and feeding habits of East African ruminants. Mammalia, 36, 226– 240. Kay, R. F. & Hiiemae, K. M. 1974. Jaw movement and tooth use in recent and fossil primates. American Journal of Physical Anthropology, 40, 227–256. Merceron, G., Blondel, C., Bonis, L. de, Koufos, G. D. & Viriot, L. 2005. A new method of dental microwear analysis: application to extant primates and Ouranopithecus macedoniensis (Late Miocene of Greece). Palaios, 20, 551–561. 18 Merceron, G., Blondel, C., Brunet, M., Sen, S., Solounias, N., Viriot, L. & Heintz, E. 2004. The Late Miocene paleoenvironment of Afghanistan as inferred from dental microwear in artiodactyls. Palaeogeography, Palaeoclimatology, Palaeoecology, 207, 143–163. Merceron, G., Costeur, L., Maridet, O., Ramdarshan, A. & Göhlich, U. B. 2012. Multiproxy approach detects heterogeneous habitats for primates during the Miocene climatic optimum in Central Europe. Journal of Human Evolution, 63, 150–161. Merceron, G., Schulz, E., Kordos, L. & Kaiser, T. M. 2007. Paleoenvironment of Dryopithecus brancoi at Rudabánya, Hungary: evidence from dental meso- and microwear analyses of large vegetarian mammals. Journal of Human Evolution, 53, 331– 349. Pickford, M. & Tassy, P. 1980. A new species of Zygolophodon (Mammalia, Proboscidea) from the Miocene hominoid localities of Meswa Bridge and Moroto (East Africa). Neues Jahrbuch für Geologie und Paläontologie, 4, 235–251. Sanders, W. J., Gheerbrant, E., Harris, J. M., Saegusa, H. & Delmer, C. 2010. Proboscidea. Pp. 161–251 in L. Werdelin and W. J. Sanders (eds) Cenozoic mammals of Africa. University of California Press, Berkeley, Los Angeles, London. Tassy, P. 1977. Le plus ancien squelette de gomphothère (Proboscidea, Mammalia) dans la formation burdigalienne des sables de l'Orléanais, France. Mémoires du Muséum National d'Histoire Naturelle, 37, 1–51. Tassy, P. 1985. La place des mastodontes Miocènes de l’Ancien Monde dans la phylogénie des Proboscidea (Mammalia): Hypothèses et conjectures. Unpublished PhD thesis, Université Pierre et Marie Curie, 861 pp. 19 Tassy, P. 1986. Nouveaux Elephantoidea (Mammalia) dans le Miocène du Kenya. CNRS, Paris, 135 pp. Tassy, P. 1994. Gaps, parsimony, and early Miocene elephantoids (Mammalia), with a reevaluation of Gomphotherium annectens (Matsumoto, 1925). Zoological Journal of the Linnean Society, 112, 101–117. Tassy, P. 1996. The earliest gomphotheres. Pp. 89–91 in J. Shoshani and P. Tassy (eds) The Proboscidea: Evolution and palaeoecology of elephants and their relatives. Oxford University Press, New York. Teaford, M. F. & Walker, A. 1984. Quantitative differences in dental microwear between primate species with different diets and a comment on the presumed diet of Sivapithecus. American Journal of Physical Anthropology, 64, 191–200. Vischer, N. O. E., Huls, P. G. & Woldringh, C. L. 1994. Object-Image: an interactive image analysis program using structured point collection. Binary, 6, 160–166. 20