e-m Lab peterson - Helios
advertisement

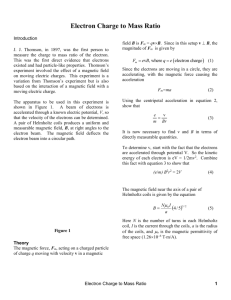
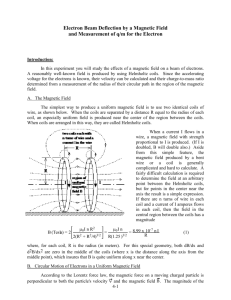
PHYS 351 Augustana College Winter 2010-2011 Experimental Determination of the Electron Charge to Mass Ratio Derick Peterson, Mitchell Anliker, Steven Ash, Augustana College, Rock Island, Illinois, USA ABSTRACT This paper presents an experimental determination of the electron charge to mass ratio (e/m) using a Helmholtz coil apparatus. The kinetic energy (determined by the voltage, V) of electrons in a beam and the current, I, in the Helmholtz coils were varied in order to change the radius of the electron beam circle in the e/m apparatus. These values were plotted, and the slope of the graph was used to calculate an experimental value for e/m (1.060x1011 +/- 3.32%). INTRODUCTION The theory of the electron made a crucial step in 1897 when J.J. Thomson was able to measure the charge to mass ratio of the electron. This was able to firmly establish that electrons existed and that they possessed particle-like properties. Thompson used the effect of a magnetic field on moving electric charges to study the behavior and properties of electrons. This experiment is a variation of Thompson’s experiment, but is also based on the interaction of a moving electric charge in a magnetic field. The experimental setup is shown below in figure 1. A beam of electrons is accelerated through a known electrical potential, V, so that the velocity of the electrons can be determined. A pair of Helmholtz coils produces a uniform measureable magnetic field, B, at right angles to the electron beam. The magnetic field deflects the electron beam into a circular path. PHYS 351 Augustana College Winter 2010-2011 Theory The magnetic force, Fm, acting on a charged particle with charge q moving through a magnetic field B with velocity v is Fm = qvB. Because velocity is perpendicular to the magnetic field in our experiment and q = e, Fm can thus be given by the equation Fm = evB (1) The electrons are moving in a circle, so they are constantly accelerating from the magnetic force, as seen in the equation Fm = ma, where a is the centripetal acceleration, a= v2/r. We can combine these relations with equation 1 to get e/m = v/Br. Each electron is accelerated through a potential V, so the kinetic energy of each electron is eV = 1/2mv2. If you use this to substitute for v, you end up with the final relation (e/m) B2r2 = 2V (2) where B is equal to the magnetic field near the axis of a pair of Helmholtz coils (3) Where N is the number of turns in each Helmholtz coil (130 in this case), I is the current through the coils, a is the radius of the coils, and µ0 is the magnetic permittivity of free space (1.26x10-6 Tm/A). Thus by using values of current, I, radius, r, and voltage, V, we can make a linear plot of various points to obtain the value of (e/m), which is the slope of the graph. METHOD The e/m apparatus manual was used to set up the experimental setup as shown below in fig. 1. The digital multimeter was used to measure the Helmholtz coil voltage. The e/m switch was flipped to PHYS 351 Augustana College Winter 2010-2011 the e/m measure position. The current adjustment knob on the Helmholtz coils was turned to OFF (care was taken that the current never exceeded 2.0A). The electric voltage V was set to 300V and the Helmholtz coil voltage was set to 8.0V. The current adjustment knob for the Helmholtz coils was then slowly turned clockwise until the electron path made a circle with a radius of approximately 5cm. The radius of the beam circle was measured at different values of voltage, V (the energy of the electrons), within the 175-300V range, and for different values of radius r, with the 4.5-5.5cm range. The values of the Helmholtz coil current, I, the voltage, V, and the radius of the electron circle, r, were recorded for each situation. The resulting data was used to plot a graph, the slope of which was used to calculate an experimental value for e/m. Figure 1. Experimental setup for e/m measurement RESULTS Table 1 below shows the experimentally determined value for e/m in comparison with its standard. This value was obtained from the slope of the Graph 1, and the uncertainty was weighted with error bars and calculated by the data analysis software. The source of error in this measurement was PHYS 351 Augustana College Winter 2010-2011 primarily in the radius squared, so that was used as the y values and the error in r2 was used for the yerror bars. The error in r, which was measured by sight, we determined to be +/- .05cm, half of the smallest measurement on the measuring stick behind the e/m bulb. The error in the current, I, we took to be .005A and the error in V as .5V, but as these values could not be expressed as error bars in the data analysis software they unfortunately were not incorporated into the final error in e/m. e/m Experimental val. (C/kg) 1.060x1011 +/- 3.32% Standard val. (C/kg) 1.76x1011 % diff 39.8% Table 1. Experimental and Standard values for e/m In order to have usable, meaningful error bars in the graph used to find e/m, equation 2 was solved for r2 such that it then becomes r2 = 2V/ B2 *1/(e/m) where by the form y=mx +b the x values are given by 2V/B2 and the slope is then 1/(e/m). Graph 1. Plot of the values of y and x given by equation 4. y= r2, x=2V/B2, and slope = 1/(e/m) (4) PHYS 351 Augustana College Winter 2010-2011 DISCUSSION The percent difference between our experimentally determined value of e/m and its standard value was 39.8%, which is well outside of the calculated error of 3.32%. Thus, our data does not fall within experimental error of the accepted value of e/m. This error is rather large, and suggests that there was a mistake somewhere along in our experiment. Of greatest concern here is in the measurement of the radius of the circular electron beam. Because the beam is inside a glass bulb it is impossible to directly measure the radius. Our method of measurement was to make readings on the reflective measuring stick placed directly behind the e/m bulb. We were told that measuring this by eye was a sufficient way of measurement, so that is the method we used. We compared the position of the outer edge of the electron beam to the measurements behind it, taking care to have the device centered in our field of vision and to take measurements from the same spot each time. However, this may have been the source of our error. In taking measurements we noticed how different members would get different readings of the same setup depending on how far or back they placed their head when reading the position. This effected the angle of the line of sight between the eye, the electron beam, and the measurement stick, and would sometimes result in members getting measurements that differed by over 1.5cm. We elected to make sure of the accuracy of our measurements by establishing a standard technique of measurement and triple-checking the measurement between members. However, it is likely that even so we were making inaccurate measurements, which compounded into our calculation of e/m through the slope of the graph. PHYS 351 Augustana College Winter 2010-2011 Additionally, it was noted that the current for the Helmholtz coils briefly went above 2.0A during some adjustments, so if this could have damaged the coils in some way, this could have introduced error into our experiment. We could improve the accuracy of this experiment by having controlled, precise adjustments over a wide range of values to get more data. More notably, we could develop some method of taking highly accurate measurements of the radius of the electron beam. References: 1. An Introduction to Error Analysis, John R. Taylor, 2nd Ed. (1997).