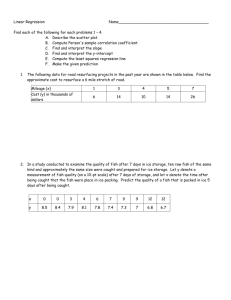
A multi-scaled approach to evaluating the fish community structure
advertisement

1 LRH: Kirsch and Peterson 2 RRH: Factors affecting fish assemblage structure 3 4 A multi-scaled approach to evaluating the fish assemblage structure within 5 southern Appalachian streams USA 6 7 Joseph E. Kirsch1 8 Warnell School of Forestry and Natural Resources, University of Georgia, Athens, Georgia, 9 30602 USA 10 11 James T. Peterson2 12 Georgia Cooperative Fish and Wildlife Research Unit, US Geological Survey, Warnell School of 13 Forestry and Natural Resources, University of Georgia, Athens, Georgia 30602 USA 14 15 1 Current address: US Fish and Wildlife Service 16 850 S. Guild Ave., Suite 105 17 Lodi, CA 95240 USA 18 E-mail address: joseph_kirsch@fws.gov 19 2 20 Current address: Oregon Cooperative Fish and Wildlife Research Unit To whom correspondence should be addressed. 21 US Geological Survey 22 104 Nash Hall, Corvallis, Oregon 97331 USA 23 E-mail address: jt.peterson@oregonstate.edu 1 24 This draft manuscript is distributed solely for purposes of scientific peer review. Its content is 25 deliberative and predecisional, so it must not be disclosed or released by reviewers. Because the 26 manuscript has not yet been approved for publication by the U.S. Geological Survey (USGS), it 27 does not represent any official USGS finding or policy. 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 2 47 48 <A>Abstract There is considerable uncertainty regarding the relative roles of stream habitat, landscape 49 characteristics, and interspecific interactions in structuring stream fish assemblages. We 50 evaluated the relative importance of environmental characteristics and species interactions on 51 fish occupancy, at local and landscape scales, within the upper Little Tennessee River Basin, 52 Georgia and North Carolina. Using a quadrat sample design, fishes were collected at 525 channel 53 units within 48 study reaches during two consecutive years. We evaluated the relative support for 54 the influence of local- and landscape-scale factors on fish occupancy using multi-scaled, 55 hierarchical, multi-species occupancy models. Modeling results suggested that the fish 56 assemblage within the Little Tennessee River Basin was hierarchically structured and primarily 57 influenced by stream size and spatial context, urban land coverage, and channel unit types. 58 Landscape scale factors (i.e., urban land coverage, stream size and spatial context) largely 59 constrained the fish assemblage structure at a stream reach and local scale factors (i.e., channel 60 unit types) influenced fish distribution within stream reaches. Our study demonstrates the utility 61 of a multi-scaled approach and the need to account for the inter-scale interactions of factors 62 influencing assemblage structure prior to monitoring fish assemblages, developing biological 63 management plans, or allocating management resources throughout a stream system. 64 65 3 66 North America possesses the richest diversity of temperate freshwater fishes within the 67 world (Jelks et al. 2008). Unfortunately, high fish endemism coupled with environmental 68 degradation has resulted in substantial imperilment among native fishes with approximately 46% 69 of the 1,187 described freshwater and diadromous fish taxa classified as vulnerable, threatened, 70 or endangered, a 92% increase from 1989 (Jelks et al. 2008). Assuming no future catastrophic 71 events, the extinction rate of North American freshwater fishes is projected to increase from 72 0.4% per decade (Ricciardi and Rasmussen 1999) to 3.2 % per decade (Burkhead 2012) due to 73 habitat deterioration associated with anthropogenic land and water development. Sedimentation, 74 nutrient loading, pollution, exotic species introduction, and altered hydrologic regimes are the 75 anthropogenic stressors that have been attributed to the precipitous increase in the freshwater fish 76 imperilment in North America (Richter et al. 1997; Ricciardi and Rasmussen 1999; Contreras- 77 Balderas et al. 2003; Dextrase and Mandrak 2006). To reduce these threats, effective 78 management strategies need to be developed at the most effective local and/or landscape levels. 79 Before these strategies are developed, it is essential to identify and quantify the primary factors 80 influencing lotic fish assemblage structure to appropriately allocate management resources 81 (Peterson and Dunham 2010). 82 The structure of a lotic fish assemblage is the result of a combination of biotic 83 interactions and environmental influences (Jackson et al. 2001). Biotic interactions (i.e., 84 primarily predation and competition) have been shown to influence assemblage composition 85 through fish species deletions (Gilliam and Fraser 2001) and by altering fish behavior, such as 86 changing habitat use (Power et al. 1985; Taylor 1996). Environmental characteristics, such as in- 87 stream hydrogeomorphology (i.e., water depth, current velocity, and substrate composition), 88 influence fish assemblage composition by providing habitats that allow certain species to persist 4 89 over others given species-specific morphological limitations and life history requirements 90 (Schlosser 1982; Moyle and Vondracek 1985; Jackson et al. 2001; Peterson and Rabeni 2001a). 91 Landscape charactersitics, such as topography and terrestrial landuse, can affect the structure of 92 local in-stream habitat characteristics, which in turn influence fish assemblage structure 93 (Schlosser 1991; Peterson and Kwak 1999; Paul and Meyer 2001). Despite the multitude of 94 studies demonstrating the effects of both biotic interactions and environmental characteristics on 95 fish assemblage structure, the percieved importance of mechanisms is not well understood and is 96 typically governed by their association with the scale of study (Jackson et al. 2001; Peterson and 97 Dunham 2010). 98 99 Scale is defined by the spatial and temporal resolution of the observational units (i.e., grain) and the dimensions of a study (i.e., extent; Peterson and Dunham 2010). All mechanisms 100 influencing fish assemblage structure, including biotic interactions and environmental 101 characteristics, potentially operate across multiple spatial and temporal scales (Figure 1; Frissell 102 et al. 1986; Tonn 1990; Poff 1997). The ability to observe or detect the effects of these factors, 103 however, depends on the within- and among-grain heterogeneity (Peterson and Dunham 2010). 104 Consequently, the perceived importance of factors influencing fish assemblage structure is 105 influenced by the spatial and temporal scale over which an investigation is conducted (Fausch et 106 al. 1994; Crook et al. 2001; Fausch et al. 2002; Peterson and Dunham 2010). For example, 107 studies conducted using large sample units in large spatial extents (e.g., stream reaches within 108 watersheds) often conclude that mechanisms operating over broad scales (e.g., topography) are 109 the primary processes affecting lotic fish assemblage structure (e.g., Fausch et al. 1994), whereas 110 studies conducted with smaller sample units and spatial extents (e.g., microhabitat use within a 111 stream reach) likely identify mechanisms operating at local levels (e.g., interspecific competition 5 112 or hydrogeomorphic characteristics) as primary processes affecting lotic fish assemblage 113 structure (Jackson et al. 2001; Peterson and Dunham 2010). Though the association between 114 spatial scale of a study and the perceived importance of factors influencing fish assemblage 115 structure have been acknowledged (e.g., Jackson et al. 2001; Quist et al. 2005), few studies have 116 elucidated the importance of biotic interactions and environmental characteristics in structuring 117 lotic fish assemblages across spatial scales. 118 The need to understand the importance of factors influencing fish assemblages is 119 exemplified by the southeastern United States. The region possesses the richest diversity and 120 greatest number of endemic fishes within North America, north of Mexico (Mayden 1988; 121 Warren et. al. 2000, Jelks et al. 2008). The Southeast also has one of the highest proportions of 122 imperiled freshwater fish taxa, where approximately 28% of the 560 described species are 123 classified as imperiled or extirpated primarily due to a variety of anthropogenic stressors 124 (Warren and Burr 1994; Warren et al. 1997; Warren et al. 2000; Jelks et al. 2008). Although lotic 125 fish assemblage structure in the Southeast has been well studied, there remains considerable 126 uncertainty as to the importance of biotic and abiotic factors across spatial scales. Therefore, we 127 evaluated the factors influencing the fish assemblage structure within a Southeast river basin 128 with the following objectives: (1) to develop multi-scale framework for evaluating the effects of 129 biotic and abiotic factors operating at geomorphic channel unit and stream reach spatial scales; 130 (2) to determine the relative importance of scale-specific effects; and (3) to quantify the scale- 131 specific relations between the most influential factors and the occupancy of multiple fish species. 132 133 6 134 135 <A>Methods Study area.- We investigated lotic fish assemblage structure and habitat use in the Little 136 Tennessee River Basin located in western North Carolina and northeast Georgia, USA. The 137 Little Tennessee River is a tributary of the upper Tennessee River and is located in the Blue- 138 Ridge province of the southern Appalachian Mountain range. The basin reportedly contains more 139 than 150 native freshwater fish species (Warren et al. 2000; Etnier and Starnes 2001). The 140 climate is classified as marine humid temperate based on its relatively mild air temperatures and 141 high moisture content (Swift et al. 1988). The Little Tennessee River Basin drains an area of 142 approximately 4,117km² and is dominated by second growth forests due to extensive 143 deforestation in 19th century (Scott et al. 2002). Anthropogenic land use within the basin 144 includes both agriculture and urban development, with agricultural land use declining and urban 145 development steadily increasing to support human immigration (Gragson and Bolstad 2006). 146 Site selection.- This study was conducted in collaboration with the Coweeta Long Term 147 Ecological Research Program's synoptic sampling and assessment project. A total of 48 sites 148 (henceforth, study reaches) were chosen to represent the range of land uses, stream sizes, stream 149 network positions, and elevations found within the Little Tennessee River Basin (Figure 2). 150 Forty-six study reaches were sampled during the pilot phase of the project in the summer (July – 151 September) of 2009. Based on the results of the pilot sampling, 8 of the 46 study reaches and two 152 additional study reaches (10 total) were selected to represent common land use (i.e., forest, 153 urban, and agriculture) and development types (i.e., valley development and hill-slope 154 development) within the Little Tennessee River Basin. The ten reaches then were sampled during 155 the spring (May) of 2010, summer (August) of 2010, and spring (May) of 2011. 7 156 We obtained riparian buffer condition data for each study reach from Long and Jackson 157 (2013). We noted if a study reach had an intact riparian buffer which was defined as fully 158 forested cover extending 10m or more on both sides of the channel. All other landscape data for 159 each study reach were calculated using readily available Coweeta Long Term Ecological 160 Research geographic information system (GIS) data (CLTER 2011) and ArcGIS software 161 version 9.3. Land use data for each study reach were derived from 2006 land cover data 162 classified from Landsat Thematic Mapper satellite imagery with 30m resolution. Land cover 163 classes were grouped into four land use classes: urban, agriculture, forest, and other. Study reach 164 elevation, gradient, and hydrologic data were derived from a United States Geological Survey 165 seamless digital elevation model with 9.353 m resolution. For each study reach, the number of 166 contributing tributaries and watersheds boundaries were delineated using protocol described by 167 Jenson and Domingue (1988). Tributaries were identified using a minimum drainage area 168 threshold of 0.0875km2 to reflect observations during the summer of 2009. Link magnitude 169 (Link) and downstream link (D-Link) were calculated to represent study reach size and study 170 reach network positions, respectively. Link magnitude was defined as the number of contributing 171 tributaries headwater streams upstream of a study reach (Shreve 1966). Downstream link was 172 defined as the link magnitude of the next downstream confluence from a study reach (Osborne 173 and Wiley 1992). 174 Sampling design.- We used a multi-scale sample design in which samples were collected 175 from individual geomorphic channel units (henceforth, channel units) nested within study 176 reaches. At each study reach, the stream was stratified into distinct channel unit types and 177 sampled using a quadrat sample design (terminology follows Williams et al. 2002). All channel 178 units within a study reach were classified as either riffle, run, or pool following standardized 8 179 definitions (Hawkins et al. 1993; Peterson and Rabeni 2001b). Riffle channel units were, on 180 average, shallow areas with swift current velocities, surface turbulence, and tended to have 181 coarser substrate than pools or runs. Conversely, pool channel units were, on average, deep areas 182 with slow current velocities, little to no surface turbulence, and tended to have finer substrate 183 than runs or riffles. Run channel units were, on average, areas with moderate depth, moderate to 184 swift current velocities, little to no surface turbulence, and mixed substrate. 185 Two to four replicates of each of the channel unit type present at each study reach were 186 randomly selected for fish sampling and habitat measurement. During the summer of 2009, the 187 randomly selected channel units were sampled during a single visit. During the spring and 188 summer of 2010 and the spring of 2011, each randomly selected sample unit was sampled twice 189 within each season to facilitate species detection probability estimation. When sampling occurred 190 twice within a season, the time between the two sampling occasions was 5-7 days to allow fish to 191 recolonize the channel units after sampling (Peterson and Bayley 1993). 192 Fishes in each channel unit at each study reach were sampled during daylight hours with 193 Smith-Root LR 24 pulsed DC backpack electrofishers operating between 0.2 to 0.3 amperes. 194 Fish sampling began at the farthest downstream channel unit at each study reach within a 195 sampling occasion. In general, prior to electrofishing, each channel unit had a 5mm mesh seine 196 deployed across the downstream end for riffles and runs or the upstream end for pools to 197 minimize fish escape and maximize detection during sampling. The seine completely blocked off 198 the end of the sample unit and was secured to the streambed. Fishes then were then sampled via 199 electrofishing using a single pass starting from the opposite end of the channel unit from where 200 the seine was deployed. In streams with average wetted widths ≤ 4 meters, one backpack 201 electrofisher was used by one crewmember with at least one additional crewmember collecting 9 202 stunned fish with a 5mm mesh dipnet. In streams with average wetted widths > 4 meters, two 203 backpack electrofishers were used and at least two additional crewmembers collected stunned 204 fish with 5mm mesh dipnets. To maximize detection while sampling, all crewmembers involved 205 with electrofishing attempted to disturb the substrate with their feet to limit stunned fishes from 206 becoming lodged on the substrate or woody debris to maximize detection. After the single pass 207 was completed for each channel unit, fishes were collected from the seine and all dipnets. 208 Immediately after sampling each channel unit, all captured fishes were identified to 209 species and released back into the stream. Fishes that could not be accurately identified in the 210 field were initially preserved in a 10% formalin solution and brought back to the laboratory. 211 Preserved fishes were later transferred to a 70% ethanol solution and identified to species. 212 Physical habitat measurements.- Water quality and physical in-stream habitat 213 characteristics expected to influence fish occupancy or the ability to detect fish were measured at 214 each study reach and channel unit in conjunction with fish sampling. Prior to fish sampling, 215 water quality characteristics were measured in flowing water using calibrated meters at each 216 study reach for each sampling occasion. Turbidity was measured using an Oakton T-100 meter to 217 the nearest 0.01 Nephelometric Turbidity Unit (NTU). Specific conductance was measured using 218 an Oakton CON 400 Series meter to the nearest 0.01 microsiemens per centimeter (μs·cm-1). 219 Immediately after fish sampling, physical in-stream habitat characteristics were estimated 220 within each channel unit at each study reach for each sampling occasion. Mean wetted width, 221 mean water velocity, and mean water depth were estimated by averaging 3-5 randomly placed 222 measurements within each channel unit (following Peterson and Rabeni 2001b). Water depths 223 were measured to the nearest centimeter using a two meter top-set wading rod. Water velocities 224 were measured at 0.6 depth using a Marsh McBirney model 2000 Flo-mate meter. The length 10 225 and wetted widths of each unit were measured to the nearest centimeter using a standard 226 measuring tape. The mean cross-sectional area of each channel unit was estimated by 227 multiplying the channel unit mean width by the channel unit mean depth. The surface area of 228 each channel unit was estimated by multiplying a channel unit mean width by length. 229 Substrate composition within a channel unit was visually estimated by two or more 230 crewmembers and averaged (Peterson and Rabeni 2001b). Substrate composition was 231 categorized based on particle diameter as fine sediment (<5mm), gravel (5-50mm), and coarse 232 sediment (>50mm; modified from Dunne and Leopold 1978). Wood density was estimated by 233 counting the pieces of wood within the wetted channel unit that were at least 50cm in length and 234 10cm in diameter or aggregates of smaller pieces of wood with comparable volume and dividing 235 by the channel unit area. 236 Statistical analysis.- The probability of detecting lotic fish species is rarely 100% and is 237 often related to the factors that influence fish populations (Bayley and Peterson 2001; Peterson 238 and Paukert 2010). To minimize the effect of incomplete species detection, we evaluated the 239 relative importance of biotic interactions, in-stream habitat, and landscape characteristics on fish 240 assemblage structure using multi-species, multi-scale occupancy models (Mordecai et. al. 2011). 241 Multi-scale occupancy models consist of three submodels that estimate the probability that a 242 species occupies a study reach (ψ), the probability that the species uses a channel unit within an 243 occupied study reach (θ), and the probability of detecting a species given it occupies a channel 244 unit (p) in a single modeling framework. All of the multi-scale occupancy submodels (i.e., ψ, θ, 245 and p) were fit using logit linear functions of predictor variables (e.g., stream habitat 246 characteristics). The primary assumption of the occupancy estimator is that a species cannot 247 colonize or abandon a study reach between sample occasions (MacKenzie et al. 2006). We 11 248 believe that this study met this assumption because sample occasions were 5 -7 days apart within 249 a season and did not include periods of seasonal fish migration or seasonal changes in habitat use 250 (Peterson and Rabeni 2001a). 251 Fish samples that were collected from multiple channel units within individual study 252 reaches were likely statistically dependent (i.e., spatially autocorrelated). In addition, the 253 relations between physical in-stream habitat and terrestrial landscape characteristics and 254 occupancy were likely to vary among fish species. To account for dependence among repeated 255 samples at study reaches and to allow model parameters to vary among species, we used 256 hierarchical logit linear models to estimate reach and channel unit occupancy and detection 257 probability (Royle and Dorazio 2008). Hierarchical models differ from more familiar linear 258 models in that they can account for spatial dependence and variation in responses among species 259 using fixed and randomly varying parameters (Appendix A). This provided a framework for 260 modeling the relation between scale-specific occupancy (i.e., study reach and channel unit within 261 reach) and habitat characteristics for multiple species with very different habitat use patterns 262 while accounting for incomplete detection using a single model and provided the means to 263 quantify the variability among species responses. 264 Based on the complexity of the modeling approach, we fit the occupancy models using 265 Markov Chain Monte Carlo (MCMC) implemented in WinBUGS version 1.4 (Lunn et al. 2000). 266 Models were fit using 700,000 iterations with a burn-in or discarding of the first 200,000 267 iterations. The number of required iterations was estimated using the global model (i.e., 268 containing all predictor variables) and testing for convergence using the Gelman and Rubin 269 diagnostic test (Gelman and Rubin 1992). Non-informative priors were used for each parameter 270 (Kéry and Royle 2008). 12 271 We used an information theoretic approach (Burnham and Anderson 2002) to evaluate 272 the relative importance of biotic and abiotic factors on fish species occupancy in study reaches 273 and channel units within study reaches. Our primary hypotheses of interest were to evaluate the 274 relative influence biotic and abiotic factors on fish occupancy and habitat use. Secondarily, we 275 sought to determine the factors influencing species detection. Thus, we initially developed a 276 global model that contained all of the predictor variables corresponding to a priori hypotheses 277 regarding the relative effect of physical and biotic factors on fish occupancy at channel unit and 278 study reach scales (Table 1). Using the global model, we then evaluated the relative fit detection 279 models that related species-specific detection to variables that could affect capture efficiency 280 including: the number of backpack electrofishers, surface area and cross sectional area of the 281 channel unit sampled, conductance, turbidity, wood density, mean water velocity, and coarse 282 substrate composition (Bayley and Peterson 2001; Peterson et al. 2004; Peterson and Paukert 283 2009; Hense et al. 2010; Price and Peterson 2010). The best approximating detection model was 284 used to evaluate the relative support for the primary hypotheses of interest. 285 Based on previous investigations (Larson and Moore 1985; Freeman et al. 1988; 286 Grossman et al. 1998; Harding et al. 1998; Jones et al. 1999; Scott 2006; Burcher et al. 2008; 287 Hazelton and Grossman 2009), we hypothesized that species occupancy at the study reach level 288 was influenced by seasonal habitat availability, stream size and spatial context, land cover, and 289 species interactions operating at reach scales (Table 1). Within occupied reaches, we 290 hypothesized that species channel unit use was influenced by the characteristics of the channel 291 unit and the presence of potential competitors. The hypothesized biotic interactions focused on 292 previously documented interactions between nonnative or invasive species and native species in 293 the Little Tennessee River Basin under laboratory conditions (Fausch and White 1981; Larson 13 294 and Moore 1985; Hazelton and Grossman 2009). Native Brook Trout (Salvelinus fontinalis) were 295 hypothesized to alter their habitat use and distribution in the presence of a nonnative Rainbow 296 Trout (Oncorhynchus mykiss) and Brown Trout (Salmo trutta). Native Rosyside Dace 297 (Clinostomus funduloides) were hypothesized to alter their habitat use and distribution in the 298 presence of the invasive Yellowfin Shiner (Notropis lutippinis). To estimate the potential effects 299 of species interactions under incomplete species detection, we used the state space formulation of 300 the occupancy model (MacKenzie et al. 2006; Mordecai et. al. 2011). Here, we used the species- 301 specific latent variables to estimate three predictor variables: the presence of the potential 302 competitor in a study reach and a channel unit within a study reach and the proportion of specific 303 channel unit types in a reach that were occupied by the potential competitor (Table 1). 304 Prior to constructing the candidate models, we estimated Pearson correlation coefficients 305 between all pairs of potential predictor variables. Only uncorrelated variables (r²<0.45) were 306 included in the same candidate occupancy models to avoid multicollinearity. All continuous in- 307 stream habitat and landscape data were standardized with a mean of zero and standard deviation 308 of one to facilitate model fitting. We also created binary indicator variables for channel unit type, 309 summer season, intact riparian buffer, and for instances where two shockers were used to sample 310 fish. We determined the best variance structure (i.e., the parameters that needed to vary 311 randomly among species or study reaches) by evaluating the relative fit of the global model with 312 each parameter systematically treated as a fixed or randomly varying effect. The best 313 approximating variance structure was identified as that with the lowest Akiake Information 314 Criteria with small sample bias adjustment (AICc; Hurvich and Tsai 1989). The number of 315 parameters used to calculate AICc included both fixed and random effects. The best 14 316 approximating variance structure then was used during the evaluation of the relative plausibility 317 of the candidate models. 318 The relative support of each candidate occupancy model was evaluated by calculating 319 AICc. Because MCMC methods produce a distribution of AICc values, we used the mean AICc 320 from MCMC iterations for inference (following Fonnesbeck and Conroy 2004). The relative fit 321 of candidate models were determined by calculating Akaike weights (wi; Burnham and Anderson 322 2002) that range from 0-1, with the greatest weight being the best fitting model. To assess the 323 amount of evidence one candidate model had over another, we calculated the ratios of Akaike 324 weights (following Burnham and Anderson 2002). Model selection uncertainty was expressed by 325 creating a confidence model set. Any candidate model with Akaike weights that were within 326 12% of the best-approximating candidate model’s Akaike weight was included within the 327 confidence set of models (following Royall 1997). All inferences were based on the confidence 328 set of occupancy models. 329 To aid interpretation of parameter estimates, we calculated odds ratios (OR) for each 330 fixed effect parameter in the confidence set of occupancy models (Hosmer and Lemeshow 2000). 331 Because the continuous data were standardized, an OR corresponded to a one standard deviation 332 increase in the corresponding predictor variable. The precision of fixed effect parameter 333 estimates was assessed by calculating 95% credible intervals that are analogous to 95% 334 confidence intervals (Congdon 2001). In general, credible intervals that contained zero were 335 considered imprecise. We also plotted empirical Bayes estimates (Royle and Dorazio 2008) of 336 the relationship between environmental characteristics and occupancy for common species. 337 338 <A>Results 15 339 A total of 525 channel units were sampled in the 48 study reaches with 183 (35%) 340 channel units classified as a pool, 200 (38%) as riffles, and 142 (27%) channel units as runs. Half 341 of the study reaches (24) contained all three channel unit types and seven reaches consisted of a 342 single habitat type. The latter group was primarily small headwater streams with link magnitudes 343 less than 10. The study sites represented a wide range of stream sizes, physical characteristics, 344 and land uses typical of the region (Table 2). The three land use types, however, were relatively 345 strongly correlated (r2 > 0.6), which prevented their inclusion in the same occupancy model. 346 Therefore, the evaluation of the effect of land use on fish community structure included only 347 urban land use because land conversion to urban use was a management concern within the 348 basin. 349 We captured 36 species during the study (Table 3). The most commonly detected species 350 were Mottled Sculpin (Cottus bairdii) and Creek Chub (Semotilus atromaculatus), which were 351 collected at more than half of the study reaches (Table 3). In general, few large bodied 352 piscivorous fishes (e.g., Smallmouth Bass Micropterus dolomieu) were collected. No species 353 were collected at all of the study reaches and no (zero) fishes were collected in five study 354 reaches. Eight species were collected in only 1-2 study reaches and less than 1% of samples 355 (Table 3). We were concerned that the occupancy model parameter estimates for these rare 356 species would be biased due to insufficient information and the effects of parameter shrinkage 357 that occurs in hierarchical models. Therefore, we eliminated these eight species from the dataset 358 and fit models using the remaining 28 species (Table 3). 359 Detection modeling.- The best approximating species detection model contained the 360 number of backpack electrofishers used, channel unit current velocity, surface area, and a 361 species-specific random effect. The Akaike weights indicated that the model was more than 33 16 362 times better supported than the next best approximating conditional detection model that 363 contained only current velocity, surface area, and a species-specific random effect. The 364 parameter estimates for the best fitting detection model indicated that the probability of detecting 365 a species was positively related to increases in channel unit surface area and negatively related to 366 the use of two backpack electrofishers and increases in current velocity (Table 4). The species- 367 specific random effect also indicated that detection varied among fish species and was, on 368 average, greatest for commonly collected species including Mottled Sculpin, Creek Chub, and 369 Blacknose Dace (Rhinichthys atratulus; Table 4; Figure 2). Therefore, the best fitting detection 370 model was used during model fit and evaluation of occupancy. 371 Occupancy modeling.- The best approximating occupancy model (AICc = 5055) 372 contained season, stream size and spatial context (i.e., elevation, gradient, link magnitude, and 373 downstream link magnitude), and land cover (i.e., urban cover and riparian buffer) for predicting 374 study reach occupancy, and channel unit type (i.e., riffle, run, and pool) and interactions between 375 channel unit type and seasons for predicting conditional channel unit occupancy (Table 4). There 376 was little or no support for any other candidate occupancy model and thus there was little support 377 for other predictors of occupancy. The best fitting model was 12.2 times better supported than 378 the next best approximating candidate model (AICc = 5060), which was similar but contained 379 study reach interference competition (interactions between the % of pools occupied by yellowfin 380 shiner or non-native salmonids and occupancy of rosyside dace or brook trout, respectively) for 381 predicting study reach occupancy. As a result, we based all inferences on the best model. 382 The best approximating model estimated that occupancy was greatest for all species in 383 larger and lower gradient streams (Table 5). In contrast, the effect and relative importance of 384 urban cover, elevation, and channel unit type on occupancy varied among species (Figures 3 and 17 385 4). Parameter estimates suggested that, on average, fish species were 2.26 times less likely to 386 occur in pool channel types relative to run channel types during the summer compared to the 387 spring (Table 5) and that the small benthic Mottled Sculpin, Central Stoneroller (Campostoma 388 anomalum), Gilt Darter (Percina evides) had affinities for riffle channel types whereas other 389 species generally had affinities for pool or run channel types (Figures 3 and 4). However, the 390 relative importance of channel type on the within-reach distribution was greater at more optimal 391 study reach conditions for each species (Figures 3 and 4). On average, fish occupancy was higher 392 as urban cover increased within a watershed for Blacknose Dace and White Suckers (Catostomus 393 commersonnii) but was negatively related to high urban cover for all other species including the 394 highland endemic Longnose Dace (Rhinichythys cataractae), Tennessee Shiner (Notropis 395 leuciodes), and Warpaint Shiner (Luxilus coccogenis; Figure 3). On average, the occupancy of 396 the warmwater intolerant Longnose Dace, Brook Trout, and Brown Trout increased with 397 elevation, whereas site occupancy of all other species decreased with elevation (Figure 4). The 398 occupancy of all fish species were, on average, 13.9 times less likely when an intact riparian 399 buffer existed (Table 5) but the highland endemic Mottled Sculpin, Longnose Dace, and 400 Rosyside Dace were, on average, 5.56 times more likely to occur when an intact riparian buffer 401 existed. 402 403 404 <A>Discussion We found that stream size and spatial context, land cover, and channel unit types were 405 the most important factors affecting fish occupancy within the Little Tennessee River Basin. 406 However, the relative importance of these factors varied among species and spatial context. We 407 surmise that environmental stability, habitat quality, and the thermal limitations of fishes largely 18 408 controls the fish assemblage structure among stream reaches, but the behavior aimed at 409 minimizing interspecific interactions and exploiting food resources affects occupancy within 410 stream reaches. Therefore, to appropriately identify the primary mechanisms controlling a lotic 411 fish assemblage requires an understanding of the local and landscape scale processes and the 412 recognition that the importance of these processes on fish assemblage structure vary with scale. 413 The structure of the fish assemblage in a stream reach is influenced, in part, by thermal 414 regimes (Jackson et al. 2001; Wehrly et al. 2003; Quist et al. 2005). The thermal regime of a 415 stream reach often varies in response to elevation, longitudinal position, and riparian cover 416 within a stream network (Scott et al. 2002; Martin and Petty 2009). In this study, the occupancy 417 of warm water intolerant fishes was positively related to elevation, whereas the occupancy of 418 warm water tolerant fishes (i.e., catastomids, centrarchids, percinids, and most cyprinids) was 419 negatively related to elevation. Scott et al. (2002) demonstrated that stream reaches within the 420 upper Tennessee River Basin have generally cooler summer mean temperatures at higher 421 elevations and this is consistent with our observations. In general, warmer streams have greater 422 productivity relative to cooler streams and fishes have greater metabolic rates in warmer water 423 habitats (Vannote et al. 1980; Schlosser 1982; Huryn and Wallace 2000). As a result, fishes that 424 can physiologically tolerant warm water often exploit the available resources in streams at lower 425 elevations (Rahel and Hubert 1991; Wehrly et al. 2003). However, the distribution of fishes that 426 cannot physiologically tolerate warm water for sustained periods of time (e.g., Brook Trout) 427 often are restricted to cooler reaches at higher elevations (Swift and Messer 1971; Vannote et al. 428 1980; Rahel and Hubert 1991; Jackson et al. 2001; Wehrly et al. 2003; Martin and Petty 2009). 429 Therefore, we hypothesize that the differences in fish distribution among stream reaches within 19 430 the Little Tennessee River Basin were, in part, influenced by the physiological limitations of 431 fishes in relation to thermal regime. 432 Fish assemblage structure also is influenced, in part, by the size, habitat complexity, and 433 stability of stream reaches (Gorman and Karr 1978; Schlosser 1982; Peterson and Rabeni 2001a; 434 Shea and Peterson 2007). We observed that fish occupancy was positively related to link 435 magnitude and negatively related to gradient within the Little Tennessee River Basin. Headwater 436 reaches typically possess relatively shallow and smaller habitats with less structural diversity 437 (e.g., substrate, depth, and current) compared to reaches farther downstream (Gorman and Karr 438 1978; Vannote et al. 1980; Schlosser 1982). In this study, we observed that headwater reaches 439 were dominated by either riffles, runs, or pools, whereas larger stream reaches typically 440 contained all of these habitats. Stream reaches with more structural diversity often support more 441 diverse fish assemblages based on possessing a broad range of habitats that allows most species 442 to encounter favorable or optimal habitats within a reach to permit persistence (Gorman and Karr 443 1978; Vannote et al. 1980). In addition, headwater reaches often are subjected to greater 444 hydrologic variability and disturbance frequency (e.g., floods and droughts) compared to more 445 downstream reaches, which can exclude or eliminate intolerant fish species without adjacent 446 source populations (Whiteside and McNatt 1972; Gorman and Karr 1978; Schlosser 1982; Resh 447 et al. 1988; Reice et al. 1990; Osborne and Wiley 1992). The negative relation between gradient 448 and fish occupancy was likely due to higher gradient streams being dominated by habitats 449 containing faster current velocities including cascades and riffles. These habitats are often 450 energetically costly (Facey and Grossman 1990) and may inhibit movement and thus 451 immigration or recolonization (Taylor and Warren 2001; Grenouillet et al. 2004). Therefore, we 20 452 hypothesize that the fish distribution among stream reaches was, in part, influenced by the 453 variation in habitat complexity and stability of a stream. 454 Anthropogenic land use activities occurring within a watershed can greatly modify the 455 processes that create and maintain the physical and chemical stream characteristics (Schlosser 456 1991; Allan 2004). Modeling results suggested that only fishes endemic to the southern 457 Appalachian highlands (i.e., those adapted to cool, sediment free, and nutrient-poor 458 environments) responded favorably to intact riparian buffers and all fishes endemic to the 459 southern Appalachian highlands were less likely to occur in watersheds with greater 460 urbanization. These results are generally consistent with previous studies of stream fishes (e.g., 461 Jones et al. 1999; Rahel 2000; Scott and Helfman 2001; Sutherland et al. 2002; Miltner et al. 462 2004; Scott 2006). Many of these studies also demonstrated that urban land use can affect fish 463 assemblages through nutrient enrichment, the introduction of contaminants, increased 464 sedimentation, and altered flow and temperature regimes. Deforestation, the loss of a riparian 465 zone, and increases in impervious cover (e.g., compacted soil, roads, and buildings) associated 466 with urbanization can cause a reduction in evapotranspiration and infiltration rates and thus 467 increase the amount of surface runoff into streams during rainfall events resulting in lower base 468 flows and more frequent peak flows (Dunne and Leopold 1978; Webster et al. 1992; Paul and 469 Meyer 2001; Allen 2004; Alberti et al. 2007). In addition, streams with little or no shading from 470 riparian cover receive greater amounts of solar radiation resulting in higher temperatures and 471 larger daily fluctuations in temperature (Swift and Messer 1971; Pusey and Arthington 2003; 472 Moore et al. 2005; Long and Jackson 2013). Urban land use practices and the loss of an intact 473 riparian buffer also can increase inputs of nutrients (e.g., industrial fertilizer and terrestrial plant 474 material), point sources (e.g., municipal discharge), and non-point sources (e.g., agricultural 21 475 pesticides) within streams, affecting water quality (Wiley et al. 1990; Webster et al. 1992; Paul 476 and Meyer 2001; Pusey and Arthington 2003; Miltner et al. 2004). Increased surface runoff 477 coupled with exposed soils created by the removal of terrestrial vegetation also increases surface 478 erosion and sediment inputs into streams (Webster et al. 1992; Harding et al. 1998; Jones et al. 479 1999; Paul and Meyer 2001; Sutherland et al. 2002). As sediment inputs increase within southern 480 Appalachian streams, aquatic habitats become more homogenous (Jones et al. 1999; Scott and 481 Helfman 2001; Sutherland et al. 2002). Overall, these anthropogenic stream modifications can 482 result in the loss and degradation of aquatic habitats, facilitating the loss of endemic fish species 483 and the homogenization of stream fish assemblages (Jones et al. 1999; Sutherland et al. 2002; 484 Miltner et al. 2004; Scott 2006). Although our study cannot identify the relative importance of or 485 differentiate between these mechanisms, the lack of evidence for an interaction between the 486 effect of urban land use and the presence of an intact riparian buffer on fish occupancy suggests 487 that intact riparian buffers alone were insufficient to mitigate the effects of large scale urban land 488 use. 489 Geomorphic channel unit types (e.g., riffle, run, or pool) represented unique, predictable 490 combinations of water depth, current velocity, and substrate composition. Because fish 491 distributions within a stream reach are known to be related to these habitat characteristics, fish 492 species may use one geomorphic channel unit type over another to fulfill one or more life history 493 requirements (Peterson and Rabeni 2001a, 2001b). Presumably, the primary objective of an 494 individual fish is to maximize survival. Fish vulnerability to terrestrial predation (e.g., birds and 495 mammals) is a function of water depth and body size with predation risk being greater for larger 496 fish in shallower habitats, whereas aquatic predation risk is generally higher for smaller fishes in 497 deeper habitats (Werner et al. 1983; Harvey and Stewart 1991). The habitat use of most fishes in 22 498 our study sites was greater in deeper channel units (i.e., pools), which suggests that risk of 499 aquatic predation may have been lower than terrestrial predation. Indeed, few large bodied 500 piscivorous fishes were collected, so the risk of aquatic predation was likely very small 501 throughout our study sites. Therefore, we hypothesize that the distribution of fish within the 502 stream reaches was to minimize risk of terrestrial predation. 503 Fishes also should attempt to maximize net energy gain for growth when selecting 504 habitats within a stream reach (Hill and Grossman 1993). Fishes should attempt to minimize 505 their energy expenditures and maximize energy inputs to maximize growth (Facey and Grossman 506 1990; Facey and Grossman 1992; Hill and Grossman 1993). High current velocity habitats 507 contain relatively high densities of macroinvertebrates (Huryn and Wallace 1987), but can be 508 energetically demanding for fishes that are not behaviorally, morphologically, or physiologically 509 adapted to high current velocity habitats (Facey and Grossman 1990; Facey and Grossman 1992; 510 Hill and Grossman 1993). Consistent with previously cited studies, we observed that most small 511 benthic fishes (e.g., Mottled Sculpin) tended to use channel units with higher current velocities, 512 whereas most pelagic fishes (e.g., sunfish) tended to use habitats with lower current velocities. 513 Small benthic fishes often possess the hydrodynamic adaptations, including a depressed or 514 streamlined body profile, modified fins, and a reduced swim bladder to limit buoyancy (Etnier 515 and Starnes 2001), which reduce the energetic costs of occupying high current habitats that have 516 high prey densities (Facey and Grossman 1992; Peterson and Rabeni 2001a). Therefore, we 517 hypothesize that fish distribution within a reach was, in part, influenced by morphological 518 constraints and fish behavioral adaptations intended to maximize survival and growth within 519 stream reaches. 23 520 In general, the strength of the relation between channel unit types and fish occupancy was 521 greater in larger and lower gradient streams and where fish species experienced less constraining 522 landscape elevations and urban coverage. As more species are added to a community, there can 523 be a corresponding increase in resource specialization (i.e., niche packing) to reduce niche 524 overlap and presumably, competition (Pianka 1974). Peterson and Rabeni (2001a) reported that 525 fish species used a smaller variety of channel unit types in Ozark stream reaches when the 526 assemblage contained more species and hypothesized that this was due to increased resource 527 specialization. Therefore, fishes may have occupied particular channel unit types in larger and 528 lower gradient streams, in part, to minimize competition. We also observed that the relation 529 between channel unit types and fish occupancy of southern Appalachian highland endemic and 530 warm water intolerant fishes was greatest as urban cover and elevation decreased, respectively. 531 We surmise that landscape scale factors controlled the fish assemblage structure among our 532 study reaches and that the distribution of fishes within reaches was the result of fish behavior 533 (e.g., habitat use, species interactions) and channel unit characteristics. 534 Management recommendations and application.- The allocation of fishery management 535 resources is generally based on the availability of resources and the perceived importance of 536 biotic and abiotic processes on fishes of concern. Fish resource managers typically identify the 537 relative importance of environmental variables and processes using data to relate the differences 538 within a fish assemblage to environmental factors. However, the spatial scale of fish surveys 539 used to collect fish data can vary considerably. Within lotic systems, surveys are often conducted 540 on stream reach or channel unit scales. If natural resource managers do not account for hierarchal 541 theory (Frissell et al. 1986; Poff 1997) and inter-scale interactions of factors within their sample 542 design and subsequent analyses, the prioritization of environmental variables and their processes 24 543 influencing fish communities can be inaccurate, which can have both biological and economic 544 consequences (Crook et al. 2001; Peterson and Dunham 2010). In this study, our multi-scaled 545 framework permitted us to take into account the incomplete detection of fishes, the hierarchical 546 nature of lotic fish assemblages, the effect of scale, and allowed for the identification of the 547 relative importance of biotic and abiotic processes on fish assemblage structure at the stream 548 reach and channel unit scales. 549 Our data indicate that the fish assemblage within the Little Tennessee River Basin was 550 hierarchically structured and primarily influenced by stream size and spatial context, 551 urbanization, and channel unit types. We found that landscape scale processes (e.g., elevation) 552 largely constrained fish distribution throughout much of the stream network and that local scale 553 processes (e.g., channel unit types) constrained fish distribution when suitable landscape 554 conditions existed for each species. These results demonstrate the utility of a multi-scaled 555 approach and the importance of accounting for inter-scale interactions prior to monitoring fish 556 assemblages, developing biological management plans, or allocating management resources 557 throughout a stream system (Frissell et al. 1986; Tonn 1990; Fausch et al. 1994; Poff 1997; 558 Scheurer et al. 2003; Quist et al. 2005). 559 Currently, there are over seven species of concern that inhabit the Little Tennessee River 560 Basin (e.g., Brook Trout and Rosyside Dace; Warren et al. 2000; Etnier and Starnes 2001; 561 NCWRC 2005). Given that management resources are finite, we suggest that the management of 562 fishes within the Little Tennessee River Basin and other similar southern Appalachian 563 watersheds should initially focus on the landscape or watershed scale processes for treatment or 564 research to identify the optimal spatial extents or conditions to implement effective local scale 565 treatments (e.g., channel unit restoration) if larger scale treatments are not feasible. 25 566 As the need for more accurate, efficient, and economical management approaches 567 continues to rise in conjunction with the imperilment of our freshwater fish species, studies that 568 are able to robustly assess the relative importance of variables that operate at multiple spatial and 569 temporal scales across larger extents will become more necessary. The use of our multi-scaled 570 framework can provide fish managers with a more holistic approach to the prioritization of 571 environmental processes affecting lotic fish assemblages and thus natural resource management. 572 Future studies should continue to expand on both the resolution and extent of the multi-scaled 573 approach used here. 574 575 576 <A>Ackowledgements We are indebted to many technicians, volunteers, and graduate students, including 577 Camille Beasley, Kristen Cecala, Justin Dycus, Andrea Fritts, Tiffany Kay, Jason Meador, 578 Angela Romito, Colin Shea, and Brittany Trushel. We thank J. Hepinstall-Cymerman for 579 assistance in obtaining geographical information system data, C. R. Jackson for assistance in 580 obtaining riparian buffer information, and we are grateful to M. Freeman, B. Ratajczak, and B. 581 Albanese for assistance in identifying specimens. Funding and logistical support for this project 582 was provided by the Coweeta Long Term Ecological Research project and the National Science 583 Foundation. The manuscript was improved with suggestions from C. Shea and anonymous 584 reviewers. The use of trade, product, industry or firm names or products is for informative 585 purposes only and does not constitute an endorsement by the U.S. Government or the U.S. 586 Geological Survey. This study was performed under the auspices of the Coweeta LTER master 587 animal use protocol AUP #A2009 4-074. This research was supported by the Long Term 588 Ecological Research Program to the Coweeta LTER Program at the University of Georgia (DEB- 26 589 0823293). The Georgia Cooperative Fish and Wildlife Research Unit is jointly sponsored by the 590 U.S. Geological Survey, the U.S. Fish and Wildlife Service, the Georgia Department of Natural 591 Resources, the University of Georgia, and the Wildlife Management Institute. 592 593 <A>References 594 Alberti, M., D. Booth, K. Hill, B. Coburn, C. Avolio, S. Coe, and D. Spirandelli. 2007. The 595 impact of urban patterns on aquatic ecosystems: an empirical analysis in Puget lowland 596 sub-basins. Landscape and Urban Planning 80:345-361. 597 598 599 Allan, J. D. 2004. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annual Review of Ecology Evolution and Systematics 35:257-284. 600 601 602 Bayley, P. B., and J. T. Peterson. 2001. An approach to estimate probability of presence and richness of fish species. Transactions of the American Fisheries Society 130:620-633. 603 604 605 Burcher, C.L., M.E. McTammany, E.F. Benfield, and G.S. Helfman. 2008. Fish assemblage responses to forest cover. Environmental Management 41:336-346. 606 607 608 Burkhead, N. M. 2012. Extinction rates in North American freshwater fishes, 1900-2010. BioScience 62(9):798-808. 609 610 611 Burnham, K. P., and D. R. Anderson. 2002. Model selection and inference: an informationtheoretic approach. Springer-Verlag, New York. 612 613 Congdon, P. 2001. Bayesian statistical analysis. John Wiley & Sons Inc., New York. 614 615 Contreras-Balderas, S., P. Almada-Villela, M. L. Lozano-Vilano, and M. E. Garcia-Ramerez. 616 2003. Freshwater fish at risk or extinct in Mexico. Reviews in Fish Biology and Fisheries 617 12:241-251. 27 618 619 620 CLTER (Coweeta Long Term Ecological Research). 2011. Historic landcover dataset. Available from http://coweeta.uga.edu/gisdata. 621 622 Crook, D. A., A. I. Robertson, A. J. King, and P. Humphries. 2001. The influence of spatial scale 623 and habitat arrangment on diel patterns of habitat use by two lowland river fishes. 624 Oecologia 129:525-533. 625 626 627 Dextrase, A. J. and N. E. Mandrak. 2006 Impacts of alien invasive species on freshwater fauna at risk in Canada. Biological Invasions 8(1): 13-24. 628 629 630 Dunne, T., and L. B. Leopold. 1978. Water in Environmental Planning. W. H. Freeman and Co, New York. 631 632 633 Etnier, D. A., and W. C. Starnes. 2001. The fishes of Tennessee. The University of Tennessee Press, Knoxville, TN. 634 635 Facey, D. E., and G. D. Grossman. 1990. The metabolic cost of maintaining position for four 636 North American stream fishes: effects of season and velocity. Physiological Zoology 637 63(4):757-776. 638 639 640 Facey, D. E., and G. D. Grossman. 1992. The relationship between velocity, energetic costs, and microhabitat use in four North American stream fishes. Hydrobiologia 239:1-6. 641 642 Fausch, K. D., S. Nakano, and K. Ishigaki. 1994. Distribution of two congeneric charrs in 643 streams of Hokkaido Island, Japan: considering multiple factors across scales. Oecologia 644 100(1/2):1-12. 645 646 Fausch, K. D., C. E. Torgersen, C. V. Baxter, and H. W. Li. 2002. Landscapes to riverscapes: 647 bridging the gap between research and conservation of stream fishes. American Institute 648 of Biological Sciences 52(6):483-498. 28 649 650 Fausch, K. D., and R. J. White. 1981. Competition between brook trout (Salvelinus fontinalis) 651 and brown trout (Salmo trutta) for positions in a Michigan stream. Canadian Journal of 652 Fisheries and Aquatic Sciences 38(10):1220-1227. 653 654 Fonnesbeck, C. J., and M. J. Conroy. 2004. Application of integrated Bayesian modeling and 655 Markov chain Monte Carlo methods to the conservation of a harvested species. Animal 656 Biodiversity and Conservation 27(1):267-281. 657 658 Freeman, M. C., M. K. Crawford, J. C. Barrett, D. E. Facey, M. G. Flood, J. Hill, D. J. Stouder, 659 and G. D. Grossman. 1988. Fish assemblage stability in a southern Appalachian stream. 660 Canadian Journal of Fisheries and Aquatic Sciences 45:1949-1958. 661 662 Frissell, C. A., W. J. Liss, C. E. Warren, and M. D. Hurley. 1986. A hierarchical framework for 663 stream habitat classification: viewing streams in a watershed context. Environmental 664 Management 10(2):199-214. 665 666 667 Gelman, A., and D. B. Rubin. 1992. Inference from iterative simulation using multiple sequences. Statistical Science 7(4):457-472. 668 669 670 Gilliam, J. F., and D. F. Fraser. 2001. Movement in corridors: Enhancement by predation threat, disturbance, and habitat structure. Ecology 82(1):258-273. 671 672 673 Gorman, O. T., and J. R. Karr. 1978. Habitat structure and stream fish communities. Ecology 59(3):507-515. 674 675 676 Gragson, T. L., and P. V. Bolstad. 2006. Land use legacies and the future of Southern Appalachia. Society and Natural Resources 19:175-190. 677 29 678 Grenouillet, G., D. Pont, and C. Hérissé. 2004. Within-basin fish assemblage structure: the 679 relative influence of habitat versus stream spatial position on local species richness. 680 Canadian Journal of Fisheries and Aquatic Sciences 61:93-102. 681 682 Grossman, G. D., R. E. Ratajczak, M. Crawford, and M. C. Freeman. 1998. Assemblage 683 organization in stream fishes: Effects of environmental variation and interspecific 684 interactions. Ecological Monographs 68(3):395-420. 685 686 687 Harding, J. S., E. F. Benfield, P. V. Bolstad, G. S. Helfman, and E. B. Jones. 1998. Stream biodiversity: the ghost of land use past. Ecology 95:14843-14847. 688 689 690 Harvey, B. C., and A. J. Stewart. 1991. Fish size and habitat depth relationships in headwater streams. Oecologia 87(3):336-342. 691 692 Hawkins, C. P, J. L. Kershner, P. A. Bisson, M. D. Bryant, L. M.Decker, S. V. Gregory, D. A. 693 McCullough, C. K. Overton, G. H. Reeves, R. J. Steedman, and M. K. Young. 1993. A 694 hierarchical approach to classifying stream habitat features. Fisheries 18(6):3-12. 695 696 Hazelton, P. D., and G. D. Grossman. 2009. Turbidity, velocity and interspecific interactions 697 affect foraging behaviour of rosyside dace (Clinostomus funduloides) and yellowfin 698 shiners (Notropis lutippinis). Ecology of Freshwater Fish 18(3):427-436. 699 700 Hense, Z., R. W. Martin, and J. T. Petty. 2010. Electrofishing capture efficiencies for common 701 stream fish species to support watershed-scale studies in the Central Appalachians. North 702 American Journal of Fisheries Management 30:1041-1050. 703 704 705 Hill, J., and G. D. Grossman. 1993. An energetic model of microhabitat use for rainbow trout and rosyside dace. Ecological Society of America 74(3):685-698. 706 707 708 Hosmer, D. W. Jr., and S. Lemeshow. 2000. Applied logistic regression. John Wiley & Sons Inc., New York. 30 709 710 711 Hurvich, C. M., and C. Tsai. 1989. Regression and time series model selection in small samples. Biometrika 76:297-307. 712 713 714 Huryn, A. D., and J. B. Wallace. 1987. Local geomorphology as a determinant of macrofaunal production in a mountain stream. Ecology 68(6):1932-1942. 715 716 Jackson, D. A., P. R. Peres-Neto, and J. D. Olden. 2001. What controls who is where in 717 freshwater fish communities - the roles of biotic, abiotic, and spatial factors. Canadian 718 Journal of Fisheries and Aquatic Sciences 58(1):157-170. 719 720 Jelks, H. L., S. J. Walsh, N. M. Burkhead, S. Contreras-Balderas, E. Diaz-Pardo, D. A. 721 Hendrickson, J. Lyons, N. E. Mandrak, F. McCormick, J. S. Nelson, S. P. Platania, B. A. 722 Porter, C. B. Renaud, J. J. Schmitter-Soto, E. B. Taylor, and M. L. Warren. 2008. 723 Conservation status of imperiled North American freshwater and diadromous fishes. 724 Fisheries 33(8):372-407. 725 726 Jenson, S. K., and J. O. Domingue. 1988. Extracting topographic structure from digital elevation 727 data for geographic information system analysis. Photogrammetric Engineering and 728 Remote Sensing 54(11):1593-1600. 729 730 Jones, E. D., G. S. Helfman, J. O. Harper, and P. V. Bolstad. 1999. Effects of riparian forest 731 removal on fish assemblages in southern Appalachian streams. Conservation Biology 732 13(6):1454-1465. 733 734 735 Kéry, M., and J. A. Royle. 2008. Hierarchical Bayes estimation of species richness and occupancy in spatially replicated surveys. Journal of Applied Ecology 45:589-598. 736 737 Larson, G. L., and S. E. Moore. 1985. Encroachment of exotic rainbow trout into stream 738 populations of native brook trout in the southern Appalachian mountains. Transactions of 739 the American Fisheries Society 114(2):195-203. 31 740 741 Long, L. S., and C. R. Jackson. 2013. Variation of stream temperature among mesoscale habitats 742 within stream reaches: southern Appalachians. Hydrological Processes. DOI: 743 10.1002/hyp.9818. 744 745 Lunn, D. J., A. Thomas, N. Best, and D. Spiegelhalter. 2000. WinBUGS - a Bayesian modelling 746 framework: concepts, structure, and extensibility. Statistics and Computing 10(4):325- 747 337. 748 749 MacKenzie, D. L., J. D. Nichols, J. A. Royle, K. H. Pollock, L. L. Bailey, and J. E. Hines. 2006. 750 Occupancy estimation and modeling: inferring patterns and dynamics to species 751 occurrence. Academic Press, San Diego, CA. 752 753 Martin, R. W., and J. T. Petty. 2009. Local stream temperature and drainage network topology 754 interact to influence the distribution of smallmouth bass and brook trout in a central 755 Appalachian watershed. Journal of Freshwater Ecology 24(3):497-508. 756 757 758 Mayden, R. L. 1988. Vicariance biogeography, parsimony, and evolution in North American freshwater fishes. Systematic Zoology 37(4):329-355. 759 760 761 Miltner, R. J., D. White, and C. Yoder. 2004. The biotic integrity of streams in urban and suburbanizing landscapes. Landscape and Urban Planning 69(1):87-100. 762 763 Moore, R. D., D. L. Spittlehouse, and A. Story. 2005. Riparian microclimate and stream 764 temperature response to forest harvesting: a review. Journal of the American Water 765 Resources Association 41(4):813-834. 766 767 Mordecai, R. S., B. J. Mattsson, C. J. Tzilkowski, and R. J. Cooper. 2011. Addressing challenges 768 when studying mobile or episodic species: hierarchical Bayes estimation of occupancy 769 and use. Journal of Applied Ecology 48:56-66. 770 32 771 772 Moyle, P. B., and B. Vondracek. 1985. Persistence and structure of the fish assemblage in a small California stream. Ecology 66(1):1-13. 773 774 775 NCWRC (North Carolina Wildlife Resources Commission). 2005. North Carolina wildlife action plan. Raleigh, NC. 776 777 Osborne, L. L., and M. J. Wiley. 1992. Influence of tributary spatial position on the structure of 778 warmwater fish communities. Canadian Journal of Fisheries and Aquatic Sciences 779 49(4):671-681. 780 781 782 Paul, M. J., and J. L. Meyer. 2001. Streams in the urban landscape. Annual Review of Ecology and Systematics 32:333-365. 783 784 785 Peterson, J. T., and P. B. Bayley. 1993. Colonization rates of fishes in experimentally defaunated warmwater streams. Transactions of the American Fisheries Society 122(2):199-207. 786 787 Peterson, J. T., and J. Dunham. 2010. Scale and Fishery Management. Chapter 3 in W. Hubert 788 and M. Quist, editors. Inland Fisheries Management, 3rd edition. American Fisheries 789 Society, Bethesda, MD. 790 791 792 Peterson, J. T., and T. J. Kwak. 1999. Modeling the effects of land use and climate change on riverine smallmouth bass. Ecological Applications 9(4):1391-1404. 793 794 Peterson, J. T., and C. Paukert. 2009. Data conversion. Pages 195-216 in S. Bonar, W. Hubert, 795 and D. Willis, editors. Standard sampling methods for North American freshwater fishes. 796 American Fisheries Society, Bethesda, MD. 797 798 Peterson, J. T., and C. F. Rabeni. 2001a. The relation of fish assemblages to channel units in an 799 Ozark stream. Transactions of the American Fisheries Society 130(5):911-926. 800 33 801 802 Peterson, J. T., and C. F. Rabeni. 2001b. Evaluating the physical characteristics of channel units in an Ozark stream. Transactions of the American Fisheries Society 130(5):898-910. 803 804 Peterson, J. T., R. F. Thurow, and J. W. Guzevich. 2004. An evaluation of multipass 805 electrofishing for estimating the abundance of stream-dwelling salmonids. Transactions 806 of the American Fisheries Society 133:462-475. 807 808 809 Pianka, E. R. 1974. Niche overlap and diffuse competition. Proceedings of the National Academy of Sciences 71:2141-2145. 810 811 Poff, N. L. 1997. Landscape filters and species traits: towards mechanistic understanding and 812 prediction in stream ecology. Journal of the North American Benthological Society 813 16(2):391-409. 814 815 816 Power, M. E., W. J. Matthews, and A. J. Stewart. 1985. Grazing minnows, piscivorous bass, and stream algae - dynamics of a strong interaction. Ecology 66(5):1448-1456. 817 818 Price, A. L., and J. T. Peterson. 2010. Estimation and modeling of electrofishing and seining 819 capture efficiency for fishes in wadeable warmwater streams. North American Journal of 820 Fisheries Management 30:481-498. 821 822 823 Pusey, B. J., and A. H. Arthington. 2003. Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research 54:1-16. 824 825 Quist, M. C., F. J. Rahel, and W. A. Hulbert. 2005. Hierarchical faunal filters: an approach to 826 assessing effects of habitat and nonnative species on native fishes. Ecology of Freshwater 827 Fish 14:24-39. 828 829 830 Rahel, F. J. 2000. Homogenization of fish fuanas across the United States. Science 288(5467):854-856. 831 34 832 Rahel, F. J., and W. A. Hubert. 1991. Fish assemblages and habitat gradients in a Rocky 833 Mountain - Great Plains stream: biotic zonation and additive patterns of assemblage 834 change. Transactions of the American Fisheries Society 120:319-332. 835 836 837 Rahel, F. J., and R. A. Stein. 1988. Complex predator-prey interactions and predator intimidation among crayfish, piscivorous fish, and small benthic fish. Oecologia 75(1):94-98. 838 839 Reice, S. R., R. C. Wissmar, and R. J. Naiman. 1990. Disturbance regimes, resilience, and 840 recovery of animal communities and habitats in lotic ecosystems. Environmental 841 Management 14:647-659. 842 843 Resh, V. H., A. V. Brown, A. P. Covich, M. E. Gurtz, H. W. Li, G. W. Minshall, S. R. Reice, A. 844 W. Sheldon, J. B. Wallace, and R. C. Wissmar. 1988. The role of disturbance in stream 845 ecology. Journal of the North American Benthological Society 7(4):433-455. 846 847 848 Ricciardi, A., and J. B. Rasmussen. 1999. Extinction rates of North American freshwater fauna. Conservation Biology 13(5):1220-1222. 849 850 851 Richter, B. D., D. P. Braun, M. A. Mendelson, and L. L. Master. 1997. Threats to imperiled freshwater fauna. Conservation Biology 11(5):1081-1093. 852 853 Royall, R. M. 1997. Statistical evidence: a likelihood paradigm. Chapman and Hall, New York. 854 855 Royle, J. A., and R. M. Dorazio. 2008. Hierarchical modeling and inference in ecology: the 856 analysis of data from populations, metapopulations and communities. Academic Press, 857 London, UK. 858 859 Scheurer, J. A., K. D. Fausch, and K. R. Bestgen. 2003. Multiscale processes regulate Brassy 860 Minnow persistence in a Great Plains river. Transactions of the American Fisheries 861 Society 132:840-855. 862 35 863 864 Schlosser, I. J. 1982. Fish assemblage structure and function along two habitat gradients in a headwater stream. Ecological Monographs 52(4):395-414. 865 866 Schlosser, I. J. 1991. Stream fish ecology - a landscape perspective. Bioscience 41(10):704-712. 867 868 869 Scott, M. C. 2006. Winners and losers among stream fishes in relation to land use legacies and urban development in the southeastern US. Biological Conservation 127(3):301-309. 870 871 872 Scott, M. C., and G. S. Helfman. 2001. Native invasions, homogenization, and the mismeasure of integrity of fish assemblages. Fisheries 26(11):6-15. 873 874 Scott, M. C., G. S. Helfman, M. E. McTammany, E. F. Benfield, and P. V. Bolstad. 2002. 875 Multiscale influences on physical and chemical stream conditions across Blue Ridge 876 landscapes. American Water Resources Association 38(5):1379-1392. 877 878 Shea, C. P., and J. T. Peterson. 2007. An evaluation of the relative influence of habitat 879 complexity and habitat stability on fish assemblage structure in unregulated and regulated 880 reaches of a large southeastern warmwater stream. Transactions of the American 881 Fisheries Society 136(4):943-958. 882 883 Shreve, R. L. 1966. Statistical law of stream numbers. Journal of Geology 74(1): 17-37. 884 885 Sutherland, A. B., J. L. Meyer, and E. P. Gardiner. 2002. Effects of land cover on sediment 886 regime and fish assemblage structure in four southern Appalachian streams. Freshwater 887 Biology 47(9):1791-1805. 888 889 Swift, L. W., G. B. Cunningham, and J. E. Douglass. 1988. Climatology and hydrology. Pages 890 35-55 in W. T. Swank and D.A. Crossley, editors. Forest hydrology and ecology at 891 Coweeta. Ecological Studies, Volume 66, Springer-Verlag, New York. 892 36 893 Swift, L. W., and J. B. Messer. 1971. Forest cuttings raise temperatures of small streams in the 894 southern Appalachians. Journal of Soil and Water Conservation 26(3):111-116. 895 896 897 Taylor, C. M. 1996. Abundance and distribution within a guild of benthic stream fishes: local processes and regional patterns. Freshwater Biology 36(2):385-396. 898 899 900 Taylor, C. M., and M. L. Warren. 2001. Dynamics in species composition of stream fish assemblages: environmental variability and nested subsets. Ecology 82(8):2320-2330. 901 902 Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell, and C. E. Cushing. 1980. The 903 river continuum concept. Canadian Journal of Aquatic Sciences 37:130-139. 904 905 906 Tonn, W. M. 1990. Climate change and fish communities: a conceptual framework. Transactions of the American Fisheries Society 119:337-352. 907 908 909 Warren, M. L., and B. M. Burr. 1994. Status of freshwater fishes of the United States: overview of an imperiled fauna. Fisheries 19(1):6-18. 910 911 Warren, M. L., P. L. Angermeier, B. M. Burr, and W. R. Haag. 1997. Decline of a diverse fish 912 fauna: patterns of imperilment and protection in the southeastern United States. Pages 913 105-164 in G. W. Benz and D. E. Collins, editors. Aquatic fauna in peril: the southeastern 914 perspective. Southeast Aquatic Research Institute, Special Publication 1, Lenz Design 915 and Communications, Decatur, GA. 916 917 Warren, M. L., B. M. Burr, S. J. Walsh, H. L. Bart, R. C. Cashner, D. A. Etnier, B. J. Freeman, 918 B. R. Kuhajda, R. L. Mayden, H. W. Robison, S. T. Ross, and W. C. Starnes. 2000. 919 Diversity, distribution, and conservation status of the native freshwater fishes of the 920 southern United States. Fisheries 25(10):7-31. 921 922 923 Webster, J. R., S. W. Golladay, E. F. Benfield, J. L. Meyer, W. T. Swank, and J. B. Wallace. 1992. Cathchment disturbance and stream response: an overview of stream research at 37 924 Coweeta Hydrological Laboratory. Pages 231-253 in P. J. Boon, P. Calow, and G. E. 925 Petts, editors. River Conservation and Management. John Wiley and Sons, New York. 926 927 Wehrly, K. E., M. J. Wiley, and P. W. Seelbach. 2003. Classifying regional variation in thermal 928 regime based on stream fish assemblage patterns. Transactions of the American Fisheries 929 Society 132(1):18-38. 930 931 932 Werner, E. E., J. F. Gilliam, D. J. Hall, and G. G. Mittelbach. 1983. An experimental test of the effects of predation risk on habitat use in fish. Ecology 64(6):1540-1548. 933 934 Whiteside, B. G. and R. M. McNatt. 1972. Fish species diversity in relation to stream order and 935 physiochemical conditions in the Plum Creek drainage basin. American Midland 936 Naturalist 88(1):90-101. 937 938 939 Williams, B. K., J. D. Nichols, and M. J. Conroy. 2002. Analysis and management of animal populations. Academic Press, San Diego, CA. 940 38 Table 1. Hypotheses and corresponding predictor variables by scale and hypothesis categories used to estimate the study reach and conditional channel unit occupancy and detection probability of common fish species within the Little Tennessee River Basin, GA and NC. Category Predictor Hypothesis a) Study reach scale Habitat %Riffle a + %Pool a availability Stream size and The percentage of total study reach area of riffles and pools affects occupancy by influencing hydrogeomorphic habitat characteristics. Link a spatial context The link magnitude of a study reach affects occupancy by influencing aquatic habitat characteristics, habitat volume, and hydraulic regimes. D-Link a The downstream link of a study reach affects occupancy by influencing immigration and colonization rates. Elevation a The elevation of a study reach affects the occupancy of warmwater intolerant species by influencing temperature regimes. Gradient a The gradient of a study reach affects occupancy by influencing hydrodynamic characteristics and colonization rates. Land cover Urban a The percentage of urban cover within a study reach watershed affects occupancy of highland endemic species by influencing hydraulic regimes and aquatic habitat characteristics. Riparian Buffer a The presence of an intact riparian buffer throughout the study reach affects occupancy by influencing productivity, thermal regimes, and habitat diversity. Study reach %Pool Yellowfin Shiner at The percent of pools occupied by invasive yellowfin shiners within a study interference Reach* Rosyside Dace at Reach reach affects the occupancy of rosyside dace within a study reach based on 39 competition interference competition. %Pool Non-native Salmonid at The percent of pools occupied by non-native salmonids within a study reach Reach* Brook Trout at Reach affects the occupancy of brook trout within a study reach based on interference competition. Study reach Yellowfin Shiner at The presence of at least one invasive yellowfin shiner within a study reach competitive Reach*Rosyside Dace at Reach affects the occupancy of rosyside dace within a study reach based on exclusion Seasonal competitive exclusion. Non-native Salmonid at Reach The presence of at least one non-native salmonid affects the occupancy of *Brook Trout at Reach brook trout within a study reach based on competitive exclusion. Summer*Link Summer affects occupancy by reducing habitat and precludes fish from interactions occupying small streams. Summer*Elevation Summer affects occupancy by decreasing the ground water supply in higher elevations and increasing water temperature. Land cover Riparian Buffer*Urban interactions The relation between urban cover and occupancy varies when an intact riparian buffer is present. Land cover and Gradient* Non-native Salmonid The relation between non-native salmonids and brook trout occupancy varies competitive at Reach*Brook Trout at Reach with gradient. exclusion Urban* Yellowfin Shiner at The relation between invasive yellowfin shiners and rosyside dace occupancy interactions Reach*Rosyside Dace at Reach varies with urban cover. b) Channel unit scale Channel unit type Pool a + Riffle a A pool or riffle, relative to a run, affects occupancy by influencing hydrogeomorphic characteristics. 40 Channel unit Yellowfin Shiner*Rosyside The presence of at least one invasive yellow shiner within a channel unit competition Dace*Pool + Yellowfin affects the occupancy of rosyside dace within a channel units uniquely based Shiner*Rosyside Dace*Riffle on competitive exclusion and resource partitioning. Non-native Salmonid*Brook The presence of at least one non-native salmonid within a channel unit affects Trout*Pool + Non-native the occupancy of brook trout within a channel units uniquely based on Salmonid*Brook Trout*Riffle competitive exclusion and resource partitioning. c) Inter-scale Inter-scale Yellowfin Shiner at competition Reach*Rosyside Dace*Pool + Yellowfin Shiner*Rosyside Dace*Riffle Non-native Salmonid at Reach*Brook Trout*Pool + Non-native Salmonid*Brook Trout*Riffle The presence of at least one invasive yellow shiner within a study reach affects the occupancy of rosyside dace within channel units uniquely based on competitive exclusion and resource partitioning. The presence of at least one non-native salmonid within a study reach affects the occupancy of brook trout within channel units uniquely based on competitive exclusion and resource partitioning. Channel unit and Gradient*Pool + The relation between channel unit types and occupancy is mediated by gradient landscape Gradient*Riffle given changes in hydrodynamics. interactions Link*Pool + Link*Riffle The relation between channel unit types and occupancy varies with link based on decreasing variability in hydrogeomorphic characteristics among channel units as streams become larger. Seasonal channel Summer*Pool + The relation between channel unit types and occupancy varies with summer by unit interactions Summer*Riffle less flow being present and forcing fish to seek refugia in pools. 41 a The effect was modeled as varying among species. 42 Table 2. Mean, standard deviation (in parentheses), and range of environmental conditions in sampled study reaches within the Little Tennessee River Basin during the summer of 2009, spring and summer of 2010, and spring of 2011. Variable Mean (SD) Range Elevation (m) 691.8 (88.21) 611 - 1060 Gradient (%) 4.6 (5.09) 0.5 - 23.7 Link magnitude 25.1 (28.31) 1 - 116 Downstream link magnitude 68.4 (174.86) 2 - 1112 Watershed area (km2) 7.3 (8.44) 0 - 36 Riparian buffer intact (%) 46.05(50.04) 0 - 100 Urban cover (% watershed) 5.6 (8.99) 0 - 48 Pool (% study reach area) 37.2 (23.54) 0 - 100 Riffle (% study reach area) 39.6 (19.39) 0 - 100 Run (% study reach area) 23.2 (24.04) 0 - 100 26.5 (10.67) 8 - 56 Turbidity (NTU) 8.0 (4.93) 1 - 35 Surface area (m²) 39.8 (26.898) 1 - 196 Mean cross sectional area (m²) 0.94 (0.751) 0.02 - 4.9 Mean depth (m) 0.24 (0.120) 0.02 - 0.7 Mean velocity (m/s) 0.32 (0.173) 0.02 - 1.0 Coarse sediment (%) 33.72 (20.768) 0 - 90 Fine sediment (%) 38.65 (26.075) 5 - 95 0.03 (0.052) 0 - 0.4 Study reach characteristics Channel unit characteristics Conductivity (μs/cm) Woody debris (no./m²) 941 43 Table 3. Fish species, the number and percentage (in parenthesis) of sites and samples they were collected, and the total length (TL) mean and range (in parenthesis) observed within the Little Tennessee River Basin. Species Sites Samples Mean TL (mm) Mountain Brook Lamprey Ichthyomyzon greeleyi 10 (20.8) 40 (7.6) 117.6(52-154) Central Stoneroller Campostoma anomalum 19 (39.6) 94 (17.9) 94.8(34-237) Goldfish Carassius auratus 1 (2.1) 1 (0.2) 118.0(117-119) Rosyside Dace Clinostomus funduloides 18 (37.5) 87 (16.6) 65.5(28-109) Whitetail Shiner Cyprinella galactura 8 (16.7) 31 (5.9) 80.1(32-169) Warpaint Shiner Luxilus coccogenis 12 (25) 65 (12.4) 79.6(30-196) River Chub Nocomis micropogon 15 (31.2) 73 (13.9) 112.7(33-256) Golden Shiner Notemigonus crysoleucas 1 (2.1) 2 (0.4) 82.2(77-88) Tennessee Shiner Notropis leuciodes 12 (25) 50 (9.5) 64.2(33-84) Yellowfin Shiner Notropis lutipinnis 8 (16.7) 41 (7.8) 66.5(30-118) Mirror Shiner Notropis spectrunculus 2 (4.2) 3 (0.6) 68.0(64-72) Telescope Shiner Notropis telescopus 2 (4.2) 8 (1.5) 77.6(66-91) Fatlip Minnow Phenacobius crassilabrum 5 (10.4) 8 (1.5) 91.3(71-113) Fathead Minnow Pimephales promelas 1 (2.1) 1 (0.2) 64.0(N/A) Blacknose Dace Rhinichthys atratulus 17 (35.4) 85 (16.2) 55.5(23-161) Longnose Dace Rhinichythys cataractae 13 (27.1) 50 (9.5) 83.7(31-155) Creek Chub Semotilus atromaculatus 31 (64.6) 129 (24.6) 69.6(19-193) White Sucker Catostomus commersonnii 5 (10.4) 11 (2.1) 175.0(86-296) Northern Hog Sucker Hypentelium etowanum 15 (31.2) 75 (14.3) 120.0(27-335) Black Redhorse Moxostoma duquesnii 3 (6.2) 8 (1.5) 400.7(90-506) Golden Redhorse Moxostoma erythrurum 3 (6.2) 6 (1.1) 153.6(40-432) Rainbow Trout Oncorhynchus mykiss 20 (41.7) 82 (15.6) 115.1(30-405) Brown Trout Salmo trutta 7 (14.6) 31 (5.9) 138.9(31-505) Brook Trout Salvelinus fontinalis 7 (14.6) 17 (3.2) 143.3(52-362) Western Mosquitofish Gambusia affinis 1 (2.1) 1 (0.2) 43.0(N/A) Mottled Sculpin Cottus bairdii 38 (79.2) 243 (46.3) 62.5(18-121) Rock Bass Ambloplites rupestris 8 (16.7) 28 (5.3) 124.7(26-262) Redbreast Sunfish Lepomis auritus 15 (31.2) 39 (7.4) 98.6(34-183) Green Sunfish Lepomis cyanellus 5 (10.4) 13 (2.5) 70.4(34-134) Bluegill Sunfish Lepomis macrochirus 8 (16.7) 21 (4) 66.9(38-115) Smallmouth Bass Micropterus dolomieu 3 (6.2) 8 (1.5) 226.8(190-253) Spotted Bass Micropterus punctulatus 3 (6.2) 4 (0.8) 51.2(42-61) Largemouth Bass Micropterus salmoides 2 (4.2) 4 (0.8) 53.9(33-78) Greenside Darter Etheostoma blennoides 4 (8.3) 9 (1.7) 98.8(53-121) Greenfin Darter Etheostoma chlorobranchium 1 (2.1) 3 (0.6) 72.6(63-78) Gilt Darter Percina evides 3 (6.2) 19 (3.6) 57.8(44-77) 942 44 943 Table 4. Standardized parameter estimates (standard deviation in parentheses), 95% credible intervals (CI), and odds ratios (OR) for the best fitting candidate occupancy model predicting study reach occupancy (ψ), conditional channel unit occupancy (θ│ψ), and conditional detection probability (p│ψθ) for common fish species within the Little Tennessee River Basin during the summer of 2009, spring and summer of 2010, and spring of 2011. 95% CI Parameter Estimate OR Lower Upper Study Reach Occupancy (ψ) Fixed effects Intercept -4.019 (1.021) -6.004 -2.000 0.018 Link 2.673 (0.795) 1.114 4.214 14.483 D-Link -0.712 (0.550) -1.841 0.333 0.491 Elevation -2.035 (1.138) -4.301 0.165 0.131 Gradient -5.096 (1.133) -7.323 -2.871 0.006 Urban -1.035 (0.902) -2.833 0.733 0.355 Riparian Buffer -2.625 (1.229) -4.990 -0.156 0.072 Summer 0.192 (0.165) -0.126 0.518 1.212 Random effects Intercepta 4.537 (0.713) 3.189 5.854 Linka 2.739 (0.574) 1.834 4.074 1.456 (0.533) 0.629 2.699 5.008 (0.664) 3.548 5.953 5.231 (0.564) 3.928 5.970 3.116 (0.655) 2.060 4.631 D-Link a Elevation a Gradienta a Urban a Riparian Buffer Site Fixed effects Intercept Riffle Pool Summer*Riffle Summer*Pool Random effects Rifflea Poola 5.515 (0.399) 4.524 5.985 3.583 (0.635) 2.524 5.019 Conditional Channel Unit Occupancy (θ│ψ) 1.514 (0.168) -0.581 (0.715) 2.786 (0.850) -0.010 (0.260) -0.817 (0.409) 1.209 -1.905 1.265 -0.512 -1.636 1.865 0.920 4.597 0.507 -0.023 3.415 (0.822) 2.107 5.328 3.294 (0.902) 1.869 5.383 Conditional Detection Probability (p│ψθ) Fixed effects 45 4.545 0.560 16.216 0.990 0.442 Intercept Velocity Surface Area 2 Shockers Random effects 0.122 (0.273) -0.149 (0.052) 0.119 (0.055) -0.242 (0.122) -0.421 -0.25 0.012 -0.478 0.654 -0.046 0.229 -0.001 Intercepta 1.371 (0.219) 1.012 1.868 a 1.13 0.862 1.126 0.785 Random effects are expressed as variance components that varied among species. 944 46 945 Figure captions 946 947 Figure 1. Location of study reaches (n=48) within the Little Tennessee River Basin, 948 GA and NC. 949 950 Figure 2. Estimated mean conditional detection probabilities (р│ψθ) and their 95% 951 credible intervals for commonly observed fish species using a single shocker under 952 average channel unit conditions (current velocity = 0.32m/s, surface area = 39.8m2). 953 Species are listed in order of the percent of samples they were collected in. 954 955 Figure 3. Estimated mean occupancy probabilities (ψθ) for species within pool (solid line), riffle 956 (dotted line), and run (broken line) channel types in relation to urban cover (%) during the spring 957 assuming a non-intact riparian buffer, link = 87, D-link = 62, gradient = 1, and elevation = 674m 958 in the Little Tennessee River basin, GA and NC. Species with imprecise estimates of the effect 959 of urban cover on occupancy were excluded. 960 961 Figure 4. Estimated mean occupancy probabilities (ψθ) of species within pool (solid line), riffle 962 (dotted line), and run (broken line) channel types in relation to elevation (m) during the spring 963 assuming a non-intact riparian buffer, link = 87, D-link = 62, gradient = 1, and urban cover = 964 7.5% in the Little Tennessee River basin, GA and NC. Species with imprecise estimates of the 965 effect of urban cover on occupancy were excluded. 47 966 48 Species Mottled Sculpin Creek Chub Central Stoneroller Rosyside Dace Blacknose Dace Rainbow Trout Northern Hog Sucker River Chub Warpaint Shiner Longnose Dace Tennessee Shiner Yellowfin Shiner Mountain Brook… Redbreast Sunfish Whitetail Shiner Brown Trout Rock Bass Bluegill Sunfish Gilt Darter Brook Trout Green Sunfish White Sucker Greenside Darter Fatlip Minnow Black Redhorse Smallmouth Bass Golden Redhorse Telescope Shiner 0 0.2 0.4 0.6 Detection Probability 967 49 0.8 1 1 1 0.8 0.8 0.6 A) Blacknose Dace 0.6 0.4 0.4 0.2 0.2 0 0 0 0.001 12 24 36 48 0 0.8 0.0006 0.6 0.0004 0.4 0.0002 0.2 0 0 Occupancy Probability 0.0008 0 1 12 24 12 1 B) Bluegill Sunfish 36 48 1 C) Green Sunfish 0.8 0.8 0.6 0.6 0.4 0.4 0.2 0.2 24 36 48 G) Warpaint Shiner 0 0 12 24 36 48 36 48 36 48 36 48 H) White Sucker 0 0 1 12 24 36 48 0 0.8 D) Longnose Dace 0.8 12 24 I) Whitetail Shiner 0.6 0.6 0.4 0.4 0.2 0.2 0 0 0 0.05 12 24 36 E) Mountain Brook Lamprey 0 48 0.005 0.04 0.004 0.03 0.003 0.02 0.002 0.01 0.001 0 0 0 968 F) Tennessee Shiner 12 24 36 12 J) Yellowfin Shiner 0 12 48 Urban Cover (%) 50 24 24 0.001 0.0008 0.0006 0.0004 0.0002 0 650 1 0.8 0.6 0.4 0.2 0 Occupancy Probability 610 1 0.8 0.6 0.4 0.2 0 610 1 0.8 0.6 0.4 0.2 0 610 0.375 0.3 0.225 0.15 0.075 0 610 1 0.8 0.6 0.4 0.2 0 610 969 1 0.8 0.6 0.4 0.2 0 A) Bluegill Sunfish G) Longnose Dace 650 750 850 950 1050 1 H) Mottled Sculpin 0.8 0.6 B) Brook Trout 0.4 0.2 0 610 685 760 835 910 985 1060 685 760 835 910 985 1060 0.5 I) Mountain Brook Lamprey 0.4 0.3 0.2 C) Brown Trout 0.1 0 610 685 760 835 910 985 1060 685 760 835 910 985 1060 1 D) Central Stoneroller 0.8 0.6 0.4 J) River Chub 0.2 0 685 760 835 910 985 1060 610 685 760 835 910 985 1060 0.03 K) Rosyside Dace E) Creek Chub 0.025 0.02 0.015 0.01 0.005 0 610 685 760 835 910 985 1060 685 760 835 910 985 1060 0.75 F) Gilt Darter L) Yellowfin Shiner 0.6 0.45 0.3 0.15 0 685 760 835 910 985 1060 610 685 760 835 910 985 1060 Elevation (m) 750 850 950 1050 51 970 Appendix A. The structure of the hierarchical, multiscale occupancy models used to estimate 971 species presence and habitat use 972 973 The hierarchical models used to estimate study reach occupancy (ψ), the probability that the 974 species uses a channel unit within an occupied study reach (θ│ψ), and the probability of 975 detecting a species given it occupies a channel unit (p) as: 976 Logit(ψij) = α0j + α0i + α1iW1 + … + αriWr 977 Logit(θij│ψij) = γ 0 + γ1iX1 + γ2iX2 + … + γriXr 978 Logit(рijh│ψij, θij) = β0i + β 1Y1 + … + β rYr 979 where i indexes species, j indexes study reaches, h indexes replicated samples, Wr represents a 980 study reach-specific predictor variable (e.g., terrestrial landscape characteristic), α0j represents a 981 randomly varying intercept that varied among study reaches, α0i represents a randomly varying 982 intercept that varied among species, αri represents the effect of Wr on reach occupancy that varied 983 among species, Xr represents a channel unit-specific predictor variable (e.g., physical in-stream 984 habitat characteristic), γri represents the effect of Xr on channel unit occupancy that varied among 985 species, Yr represents an in-stream habitat predictor variable (e.g., water quality or physical in- 986 stream habitat characteristic), β0i represents a randomly varying intercept that varied among 987 species, and β r represents the effect of Yr on detection. Random effects (i.e., randomly varying 988 intercepts and slopes) were assumed to be normally distributed with a mean of zero and random 989 effect-specific variance (Royle and Dorazio 2008). 52