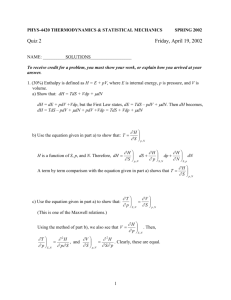
Word - Oxford Sparks
advertisement

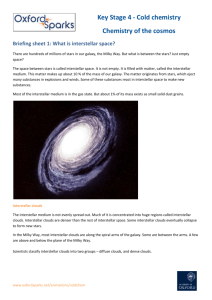
Key Stage 5 - Cold chemistry Beyond the stars Briefing sheet 1: What is interstellar space? There are hundreds of millions of stars in our galaxy, the Milky Way. But what is between the stars? Just empty space? The space between stars is called interstellar space. It is not empty. It is filled with matter, called the interstellar medium. This matter makes up about 10 % of the mass of our galaxy. The matter originates from stars, which eject many substances in explosions and winds. Some of these substances react in interstellar space to make new substances. Most of the interstellar medium is in the gas state. But about 1% of its mass exists as small solid dust grains. Interstellar clouds The interstellar medium is not evenly spread out. Much of it is concentrated into huge regions called interstellar clouds. Interstellar clouds are denser than the rest of interstellar space. Some interstellar clouds eventually collapse to form new stars. In the Milky Way, most interstellar clouds are along the spiral arms of the galaxy. Some are between the arms. A few are above and below the plane of the Milky Way. Scientists classify interstellar clouds into two groups – diffuse clouds, and dense clouds. Diffuse interstellar clouds The particles in diffuse clouds are very spread out. There are between 10 and 1000 particles in one cubic centimetre (cm3). Compare this to the Earth’s atmosphere, which has about 1019 particles/cm3. Diffuse clouds are cold, with temperatures between 50 K and 100 K (−253 ºC and −173 ºC). They are much colder than anywhere on Earth – even Antarctica, with the lowest recorded temperature of 184 K (−89 ºC). Dense interstellar clouds The particles in dense clouds are closer together than the particles in diffuse clouds. In the core of a dense cloud, there can be as many as 100 000 particles/cm3. Dense clouds are even colder than diffuse clouds. In their cores, temperatures of 10 K (−263 ºC) are common. The matter around the core is slightly warmer, with temperatures up to 25 K (−248 ºC). These low temperatures are close to absolute zero, 0 K (−273 ºC). The temperatures show that particles in interstellar clouds move very slowly indeed. Dense interstellar clouds are enormous, with diameters of several light years. Their cores have diameters of up to 1 light year. Guiding questions 1 2 3 4 What is the interstellar medium? What is an interstellar cloud? What might be formed when it collapses? Where, in our galaxy, are most interstellar clouds? Draw a table to compare diffuse interstellar clouds, dense interstellar clouds, and the Earth’s atmosphere at sea level. Include data on temperature (expressed in Kelvin) and density (expressed in number of particles/cm3). www.oxfordsparks.net/animations/coldchem Beyond the stars Briefing sheet 2: What particles are in interstellar space? There are hundreds of millions of stars in our galaxy. But what is between the stars? Just empty space? The space between stars is filled with matter. The matter is called interstellar medium. Most of the interstellar medium is concentrated into huge interstellar clouds. Scientists classify interstellar clouds into two groups – diffuse clouds, and dense clouds. Most of the interstellar medium is in the gas state. But about 1% of its mass exists as small solid dust grains. The interstellar medium is a mixture of substances. Some of these substances are new to science. They exist only in the conditions of interstellar space. The mixtures that make up the two types of interstellar clouds are different. Particles in diffuse interstellar clouds Most particles in diffuse clouds are simple atoms and molecules. Some of these are free radicals (species with unpaired electrons). Hydrogen atoms (H) and molecules (H2) are most common, then helium atoms. There are smaller amounts of oxygen atoms, nitrogen molecules, and carbon monoxide molecules. The following neutral free radicals also occur: OH, CH, CN, and NH. Scientists have also detected ions in diffuse interstellar clouds. No one has identified all the ions. Many of the ions seem exotic, since they do not exist naturally on Earth. Examples include ions with the formulae S +, HCO+, CH+, C7- and C60+. Particles in dense interstellar clouds Most gas particles in dense interstellar clouds are molecules. Amazingly, scientists have detected molecules of more than 120 substances. Many of these molecules are free radicals. The most common molecules are hydrogen and carbon monoxide, with hydrogen having a concentration roughly 1000 times greater than that of the next most abundant molecule, carbon monoxide. Other common molecules include water, ammonia and methanol. Scientists have also detected molecules of compounds that do not exist naturally on Earth, including - in order of abundance - those with the formulae C2H, C2, CN, CH, H2CO, CS, C4H, SO, HC3N, HC5N, CH3C2H, HC9N, C6H and HC11N. There are also strange molecules that include three carbon atoms joined in a ring, for example C 3H and C3H2. Some scientists report that they have found evidence that amino acids, for example glycine, exist in dense interstellar clouds. Is this evidence that the molecules of life originated in interstellar space? This is an on-going area of research. Some of the particles in dense interstellar clouds are positively-charged ions. They include H3+, HCO+ and H3O+. Scientists have detected the following atoms - 13C, 18O and 2H - in the molecules and ions of dense interstellar clouds. Interstellar dust Scientists have studied interstellar dust grains carefully. The grain diameters vary from 0.001 μm to 1 μm. Scientists have identified four groups of dust-forming elements, including (1) C and O; (2) Mg, Si, and Fe; (3) Na, Al, Ca, and Ni; (4) K, Ti, Cr, Mn, and Co. They assume that oxygen atoms are also present in the compounds that make up interstellar dust grains. Substances such as carbon monoxide, carbon dioxide and methanol gather on dust grains in their solid states, forming mixed ice mantles. Guiding questions 1 2 3 Name the most common element in the interstellar medium. Draw a dot and cross diagram of its molecule and atom. Draw a table showing the names of some of the species found in diffuse interstellar clouds. Try drawing displayed formulae, or dot and cross diagrams, to show the bonding in as many of these species as possible. For many of the species given, there are several possible answers, any of which might be correct. Repeat question 2 for the species found in dense interstellar clouds. www.oxfordsparks.net/animations/coldchem Beyond the stars Briefing sheet 3: Interstellar kinetics There are hundreds of millions of stars in our galaxy. But what is between the stars? Just empty space? The space between the stars is filled with matter. The matter is called interstellar medium. The interstellar medium is a mixture of particles of many substances. Most of the interstellar medium is in the gas state. But about 1% of its mass exists as small solid dust grains. Much of the interstellar medium is concentrated into huge regions called interstellar clouds. The particles in the interstellar medium are very spread out. In some regions, there are only 10 particles/cm 3. Compare this to the Earth’s atmosphere, which has about 1019 particles/cm3. Interstellar space is cold. Temperatures between 10 K (−263 ºC) and 100 K (−173 ºC) are common. How do conditions in interstellar space affect the kinetics of gas reactions? Chemical reactions make new substances in interstellar space, just as they do on Earth. Particles must collide before they can react. In interstellar space, particles move slowly, and they are very far apart. A particle might collide with another particle just once a fortnight. In Earth’s atmosphere, a particle may have 10 billion collisions every second. However, astronomers measure space timescales in millions of years, so there is plenty of time for collisions. Only some colliding particles actually react, on Earth and in space. Whether or not a reaction occurs depends on the energy of the colliding particles, and the activation energy of the reaction. The Arrhenius equation predicts that the rate constant, k, for a given reaction is related to the temperature by the equation lnk = lnA – Ea/RT. At 10 K, the activation energy, Ea, for most reactions is very much greater than RT. This means that the value of E a/RT is very large and negative. For this reason, all endothermic reactions, and most exothermic reactions, do not occur efficiently in interstellar clouds. However, in interstellar space, there are great numbers of ions and free radicals. The activation energy of many reactions involving these species is zero, so their reactions can be very fast in interstellar clouds. There is evidence that, at very low temperatures, free radical reactions may actually speed up. Many free radical chain reactions occur in interstellar clouds. Here is one example: H2 + cosmic ray → H2+· + e- + cosmic ray +· H2 ions react with molecular hydrogen: H2 + H2+· → H3+· + H· +· H3 ions react with neutral atoms, for example O: H3+· + O → OH+· + H2 OH+· + H2 → H2O+· + H· H2O+· + H2 → H3O+· + H· H3O+· ions dissociate when they recombine with electrons. A variety of products result, including water molecules. Hydrogen molecules are ionised by cosmic rays: Reactions on dust grains The series of reactions described above depend on one vital starting material – hydrogen molecules. How are these formed from hydrogen atoms in the interstellar medium? Imagine a hydrogen atom colliding with a piece of dust. It sticks to the surface of the dust. It may diffuse over the surface of the dust, and find another hydrogen atom. The two atoms join together. They release a little energy. This energy allows the newly-formed hydrogen molecule to move away from the dust. Other particles form on the surface of dust grains, for example OH ·, CH·, and NH·. Eventually these particles join with hydrogen atoms to form water (H2O), methane (CH4), and ammonia (NH3). Guiding questions 1 2 3 4 Explain why you might expect reactions in the interstellar medium to be much slower than reactions in Earth’s atmosphere. Explain why many gas reactions in interstellar space actually occur rather rapidly. Give an example of a free radical chain reaction that occurs in space. Write equations for each of its steps. Describe how hydrogen atoms react to make molecules on the surface of a dust grain. www.oxfordsparks.net/animations/coldchem Beyond the stars Briefing sheet 4: How do scientists study interstellar chemistry? There are hundreds of millions of stars in our galaxy. But what is between the stars? Just empty space? The space between the stars is filled with matter. The matter is called interstellar medium. Much of the interstellar medium is concentrated into regions called interstellar clouds. Scientists have found out a huge amount about the interstellar medium. They have identified many of the substances that are in it. They have found out about the kinetics of its chemical reactions. But how do scientists study chemicals, and their reactions, many light years from Earth? Emission spectroscopy Scientists use emission spectroscopy to identify the substances in dense interstellar clouds. Molecules in the interstellar medium emit electromagnetic radiation. The emissions result from electron transitions between energy levels, and from rotational or vibrational energy changes. The frequencies of these emissions are characteristic of the specific chemical substances and can be used to identify them. The emissions are mainly in the radio, microwave and infrared portions of the spectrum. Scientists use instruments such as radio telescopes to detect the emission lines. Wikipedia ‘Diffuse Interstellar Bands’. URL: http://en.wikipedia.org/wiki/Diffuse_interstellar_band [4 May 2013] Absorption spectroscopy Stars emit electromagnetic radiation. Radiation travels from a star in all directions. Some of the radiation may travel through a diffuse interstellar cloud. Atoms and ions in the dense interstellar cloud absorb radiation of certain frequencies. The atoms and ions use this energy to promote electrons from the ground state to an excited state. For most atoms and ions, the radiation frequencies absorbed are in the ultraviolet region of the electromagnetic spectrum. This radiation is absorbed by the Earth’s atmosphere, so cannot be detected from Earth. To overcome this problem, scientists make observations from space. The International Ultraviolet Explorer of the Hubble Space Telescope has provided much useful data. Spectra of Nebulae. URL: http://www.astrosurf.com/joseribeiro/Eespectros_de_nebulosas.htm [4 May 2013] Scientists analyse absorption spectra detected by space telescopes. The data provide evidence about the chemical composition of a diffuse cloud, its temperature, and its density. Scientists cannot study every interstellar cloud like this. The technique depends on there being a direct line between the illuminating star, the interstellar cloud, and the Earth. www.oxfordsparks.net/animations/coldchem Laboratory simulations Scientists also collect evidence by doing experiments. Of course, they cannot travel to the interstellar medium. So they reproduce the conditions in the laboratory. One group of scientists cools reactants to 3 K. At this temperature, they can control the position and speed of the particles. They observe them for up to 10 seconds as they react. In another experiment, Oxford scientists use a Stark decelerator to slow molecules down. The molecules move so slowly that the temperature decreases to 0.01 K. The scientists observe their behaviour at this temperature. Guiding questions 1 2 3 Describe how scientists use emission spectroscopy to identify molecules in the interstellar medium. Describe how scientists use absorption spectroscopy to identify particles in the interstellar medium. Explain why some scientists reproduce the conditions of interstellar space in the laboratory. www.oxfordsparks.net/animations/coldchem