Supplementary Information (docx 6863K)
advertisement

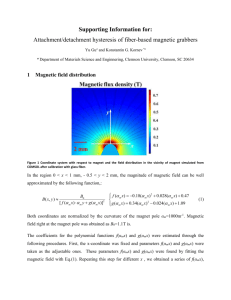
Magnetically Actuated Cell-Laden Microscale Hydrogels for Probing Strain-Induced Cell Responses in Three Dimensions Yuhui Li1,2, Guoyou Huang1,2#, Bin Gao1,2,3, Moxiao Li2,4, Guy M. Genin1,2,5,6, Tian Jian Lu2,4, Feng Xu1,2# 1 The Key Laboratory of Biomedical Information Engineering of Ministry of Education, Xi’an Jiaotong University School of Life Science and Technology, Xi’an, China 710049 2 Bioinspired Engineering and Biomechanics Center (BEBC), Xi’an Jiaotong University, Xi’an, China 710049 3 Institute of Digestive Disease, Xijing Hospital, Fourth Military Medical University, Xi’an, China 710032 4 State Key Laboratory for Strength and Vibration of Mechanical Structures, School of Aerospace, Xi’an Jiaotong University, Xi’an, China 710049 5 Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, Saint Louis, Missouri 63110, USA 6 Department of Mechanical Engineering and Materials Science, Washington University, Saint Louis, Missouri 63130, USA # Corresponding authors: fengxu@mail.xjtu.edu.cn, wwgyhuang@mail.xjtu.edu.cn Supporting Information Figure S1 (a-c) Three types of masks used for fabrication of magnetically-actuated layer (a), constrained layer (b) and cell-laden layer (c), respectively. (d) Precise size of μMAC individual element. Figure S2 (a) We minimized deformation of the magnetically-actuated layer by modifying the PEGDMA fraction. Above 15% (w/v) PEGDMA, this deformation was negligible relative to that of the GelMA layer (separation distances between the ends of the magnetic field focusers and the center of magnetically-actuated layer from 5 to 15 mm). (b-d) Shown are nominal strains in the magnetically-actuated layer influenced by the fabrication parameters. Figure S3 (a) Swelling ratio of synthetic tissue layer as a function of GelMA concentration. μMACs swelled when immersed in cell culture medium. To minimize the mechanical consequences of this, the GelMA precursor solution concentration was varied to match the swelling of the PEGDMA. (b) Swelling ratio of magnetically-actuated layer as a function of PEGDMA concentration. Figure S4 (a) XRD graph of nanoparticles, indicating the formation of pure phase Fe 3O4. (b) Size distribution histogram of as-prepared Fe3O4 nanoparticles. Figure S5 (a) Simulations showed that amplified magnetic flux density near its free end. (b) Schematic of the fields generated by applied magnet. The simulations were separated into 3 distinct sections depending on the separation of the magnetically-actuated layer from the end of the permanent magnet. (c,d) Extracted magnetic fields (c) and corresponding magnetic field gradients (d) for the different incident magnetic fields for sections III, II, and I. By symmetry, the other half of the substrate experiences forces in the order of sections I, II, and III. Figure S6 (a) The magnetic force was an approximately inverse-cubic function of this separation (symbols: experiment; lines: simulation). (b) Elastic modulus of GelMA layer could be controlled by regulating the GelMA precursor fraction. Figure S7 Magnetic loading device was constructed, each consisting of three parts for constraining μMACs (schematic (a) and objective image (b)). Scale bar: (b) 1 cm. Figure S8 (a) fibroblasts remained high viable (>90%) after encapsulation for 1 day in three kinds of μMACs. (b) The number of dead cells significantly increased in both 10 kPa and 20 kPa GelMA fraction after the 3 day culturing, indicating that cell viability is strong function of μMAC stiffness. Figure S9 (a) Live/dead fluorescence image of cells in μMACs (without magnetically-actuated layer) under external magnetic field at day 5 of culture. To rule out magnetic fields as a cause of the observed cell responses, control μMACs were constructed without iron microspheres and then placed beneath the permanent magnet at the maximum strength used in experiments for defined time intervals. (b) Cell viability analysis showed no discernable or measurable changes over 1, 3, or 5 days of culture with (+) and without (-) external magnetic field. (c) Confocal fluorescence image of cells encapsulated in μMACs with external magnetic field at day 3 of culture. (d) No statistically significant effect on cell spreading volume in μMACs with (+) and without (-) external magnetic field was observed. Scale bars: (a) 200 μm, (c) 50 μm. Figure S10 External magnetic fields showed no statistically significant effects on cellular proliferation in different culturing time. (n=10 for each data point). Figure S11. Statistical analysis of protein expression including, GAPDH, MyHC and SAA of C2C12 myoblasts in μMACs with increasing culture time. Error bars, s.d. (n=15 uMACs, **p < 0.01, ***p < 0.001). Figure S12. (a) Evaluation of degradation profile of GelMA hydrogels in culture medium. Degradation was determined by the change of hydrogel volume. (b) Quantification results of elastic modulus of GelMA hydrogel with cells (1×105 cells per ml) by increasing culture time. (n=10 for each data point).