Introduction to Spectrometry
advertisement

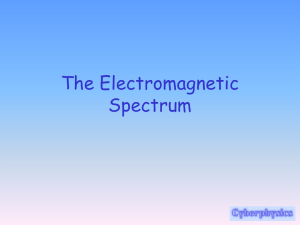
Introduction to Spectroscopy Objectives of the Unit Understand general concepts of Spectroscopy Be familiar with the common forms of spectroscopy used in molecular structure determination Be able to correctly interpret IR, 13C NMR and MS spectra Be able to use IR, 13C NMR and MS spectra to solve the structures of simple organic molecules Origins of spectroscopy Spectrum Scope The word spectrum originates with Newton's dispersion of white light into a rainbow of colours with a prism. The spectrum of white light is the range of colours that make it up. Each individual colour from the prism cannot be further separated into other colours. Each part of the spectrum has a characteristic frequency, wavelength and quantum energy. The spectrum is a continuum or range of frequency. Old definitions from a technical Dictionary: A spectroscope is a device used to observe a spectrum without the ability to record it. A spectrograph is an instrument used to photographically record a spectrum. A spectrogram is a photograph of a spectrum A spectrometer measures refractive indices A spectrophotometer is a spectroscope combined with a photometer and can measure quantitatively the relative intensity in different parts of a spectrum. These definitions are outdated as they refer primarily to visible light and older methods of recording it. All modern spectrometers ‘’look” at a spectrum and record the intensity of irradiation throughout the whole spectrum. The resulting 2 dimensional curve of wavelength or frequency VS intensity is now called a spectrum What is a spectrum? ' the colours of light separated by a prism' The visible spectrum is a small part of a much larger spectrum of electromagnetic radiation. This radiation can be thought of as transverse waves in perpendicular electric and magnetic fields: The Electromagnetic Spectrum g-rays X-rays 10-11 UV Infrared Visible light 10-7 10-9 10-5 Micro waves Radio Waves 10-1 10-3 101 103 l(m) 1Å frequency (Hz) 1mm 1nm 109 wave # (cm-1) 103 106 108 106 102 10 1011 1013 1015 1017 1019 1km 1mm 1cm 10-1 10 107 109 104 102 10 10-2 10-4 10-6 10-3 10-2 10-4 J mol -1 eV Nuclear transistions Inner electron transitions 1 J mol -1 = 2.5053 x 10 9 Hz = 8.3567 x 10 -2 cm-1 = 1.0360 x 10 -5 eV Outer electron transitions Molecular vibrations Molecular rotations 10-8 Nuclear magnetic resonance 105 Electromagnetic waves travel through transparent media (including a vacuum) at the speed of light 2.998x108 m s-1 (slightly slower through denser media). A wave passing a point in space will cause an oscillation of the electric and magnetic field. The frequency of the oscillation is related to the wavelength and speed of light. c where: c = = speed of light frequency (m s-1) (Hz) = wavelength (m) In some regions of the electromagnetic spectrum it is convenient to use wave numbers, that is, the number of wavelengths in 1 cm. The units of wave number are cm-1 and are sometimes referred to as reciprocal centimetres. Physicists near the beginning of this century began to treat electromagnetic radiation as a stream of particles. Just as matter, which seems continuous at our scale is actually made of particles at the atomic scale; so electromagnetic energy is continuous and wave-like at long wavelengths and particle like at short wavelengths. The dual wave/particle nature of electromagnetic radiation is one of the fundamental concepts of modern physics and the foundation of quantum theory. Since electromagnetic radiation comes in discrete particles, each must have a characteristic energy related to the frequency of the radiation. h where: h = = = energy Plank's constant frequency (Joules) 6.625x10-34 (J sec) (Hz) (sec-1) One can measure a spectrum in terms of wavelength, wave number, frequency, and energy. All of these units are interchangeable and all used in various forms of spectroscopy. Characteristics of a Spectrum: Intensity, Absorbency, and Abundance: (the Y axis): The Y axis in spectra measures the intensity of the radiation at a particular spot in the spectrum. In more wave-like regions of the electromagnetic spectrum this intensity may be power (energy per second). In more particle like regions photons may be counted directly. The energy may be emitted by the sample (an emission spectrum) or absorbed by the sample from a radiation source (an absorbance spectrum). In absorbance mode the transmitted beam is often compared to that present in the absence of the sample. The relative intensity is plotted on the Y axis as percent transmittance or absorbance. The advantage of plotting relative intensity is that the radiation source need not have constant intensity across the spectrum. Resolution: The resolution is a measure of the ability to discriminate close signals in the spectrum. There are a number of different ways of measuring resolution. Commonly it is defined as the width of a peak at one half of its maximum intensity. It must be remembered that in some cases the width of a peak is the natural shape of the transition, and is not a good measurement of the resolution of the spectrum. Interaction of Energy with Matter i) Classical Model Balls on Springs The oscillating electrical or magnetic field associated with electromagnetic radiation can interact with electric or magnetic moments in molecules. For example carbon monoxide is a diatomic molecule comprised of an oxygen and a carbon atom bonded together. Since the two atoms are not the same, the electrons are attracted to the more electronegative atom (oxygen) creating a dipole. The bond between the two atoms is not rigid, it can be thought of as a spring relaxed at the average inter atomic distance, compressed when the two atoms are brought closer together, and stretched when they are pulled apart. Such an arrangement (two balls on a spring) will oscillate at a natural frequency that can be described by classical physics: If this system is irradiated with electromagnetic radiation at the natural vibrational frequency, the balls will oscillate absorbing the energy. Conversely, if the system is excited it will vibrate at the natural frequency emitting the corresponding electromagnetic radiation. In general, the classical approach attempts to model reality with a single construction (balls on a spring). ii) Quantum Mechanics Model Some forms of energy/matter interactions are poorly explained by classical mechanics (i.e. electronic transitions). Classical mechanics views time, matter and energy as continuous variables. This view was modified early on at the microscopic level by the realisation that matter is made of discrete packets (atoms and subatomic particles). Time and matter are also discontinuous at the microscopic level; quantum mechanics is a modification of classical mechanics that successfully deals with the discontinuous nature of the microscopic scale. One of the fundamental outcomes of a quantum mechanical treatment of molecular energies is that the energies, be they rotational, vibrational nuclear or electronic, are quantized. This means that emissions and absorbances can only occur at specific energies, and thus specific wavelengths of electromagnetic radiation. The quantum mechanical approach uses populations of constructions to model reality. E1 Absorption Emission E0 The frequency of electromagnetic radiation absorbed or emitted is given by: n= ( E1 - E 0 ) h For most transitions the energy difference is sufficient that almost all of the population is in the lower energy state and the higher energy state can be considered unoccupied at thermal equilibrium.