Mineral HW 22 X-ray diffraction
advertisement

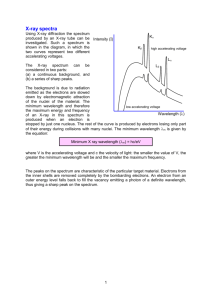
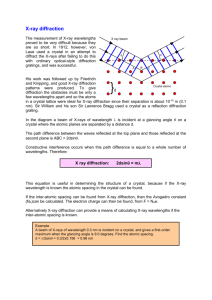
HW 10 X-Ray Powder Diffraction (unknown) I. Name _______________________________ Identification of an unknown Mineral from a X-ray powder diffractometer spacing See theory of xray generation below. X-ray Generation and Powder Diffraction 5.1 X-ray Generation Fig. 5.1 X-ray tube Illustrated above is a cross section through an X-ray tube showing the filament, target, and X-ray windows. There is a large DC potential between the filament (cathode) and the target (anode) of approximately 40,000 volts. The electrons stream off the filament and hit the target with energies equal to their charge (1e) times the potential (40KV) or 4.0 x 104 eV (electron volts). (One electron volt equals 1.602 x 10-19 joules.) Energy level diagram for Cu These energetic electrons strike the target, which is a pure metal such as copper or molybdenum, and re move inner (K) shell electrons. When this happens, other electrons from higher level shells drop into the vacant K1 shell and in so doing emit a photon (X-ray) whose wavelength (energy) is characteristic of the metal target material. In order to remove the inner shell electron, the incoming electron must have an en ergy greater than the difference in energy between the inner (K) shell electron and a free electron in the con duction band of the target metal. This energy differ ence is referred to as the absorption edge energy. The various energy levels of electrons in the target metal atoms are illustrated in Figure 5.2. The energies of the photons can be computed form the wavelengths by e = h nu = hc/lambda where h is Planck's constant (6.6 x 1034 joule-sec) c is the speed of light (3 x 108 m/sec), and Lambda is the wavelength (typically in Ångstroms, 1Å = 10-10 m). Fig 5.3. A typical spectrum of emitted X-rays from an X-ray tube is illustrated in Figure 5.3 in which intensity is plotted versus wavelength. X-ray Diffraction In X-ray diffraction, we utilize the elastic scattering of X-rays by the electrons of the atoms in a crystal. Elastic scattering is scattering in which the wavelength of the X-ray does not change, only the direction changes. Because a crystal is a periodic array of atoms with a repeat distance on the order of a few Ångstroms, it acts like a diffraction grating for photons of approximately the same wavelength as the repeat distance. As mentioned at the beginning of the section on crystal chemistry, crystals are capable of scattering X-rays in coherent patterns because X-rays have a wavelength which is within one order of magnitude of the lattice spacing. This also is true of other types of radiation (e.g. electrons and neutrons). BC = d sin q 2 Figure 5.4. X-ray diffraction geometry. The path-length difference between rays scattered from adjacent lattice planes must be an integral number of wave-lengths. Bragg diffraction occurs when a grating (crystal) scatters radiation "in phase". This happens when the exit beam is retarded by an integral number of wavelengths. This is satisfied when nlambda = 2d sin q This is known as the Bragg Equation and is used to compute the scattering angle, theta, for a given lattice spacing, d, and X-ray wavelength lambda. 3 This comes from Klein’s Minerals and Rocks Ch 22. Look at preceding 2 chapters for theory. In a powder mount finely ground mineral dust is sprinkled randomly on a Vaseline smeared slide. The crystals fall randomly. The diffractometer motor scans through ~180° less a bit so the X ray beam doesn’t burn out the detector. Pick the most intense peaks = the most common d spacings in the miieral lattice. Measure their 2 theta position and intensity relative to you highest peak. Solve the bragg equation for d spacings one at a time using Cu K alpha radiation Lambda = 1.5405 sometimes (at approptiate 2 theta) the alpha 1 and alpha 2 wavelengths disperse and can be both used for more precision in measurements. Measure 2 theta, divide by 2, take sin, multiply by 2, divide this into lambda = d. Repeat for next peak 2 theta. Tabulate your d values, arrange them most intense first, second most etc. Compare to d spacings tabulated for known minerals. Identify your unknown mineral. 4 5 6