Lab 2 - Molarity answers
advertisement

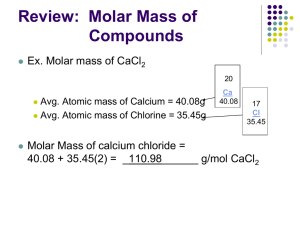
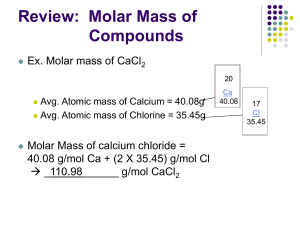
LABORATORY #2: Molarity Lab Introduction: A solution consists of one or more substances (Solutes) that are dissolved in a liquid. If the liquid if water, it is called an aqueous solution. The concentration of a solution specifies the amount of a solute dissolved in a specific volume of liquid. We can express the amount of a solute added to a liquid to make a solution in two ways 1. By weight 2. By the number of particles You can express the amount of solute by weight using ratios. Ratios indicate the proportion of solute (in grams) per volume liquid (in milliliters). For example: A vial labeled 1:1,000 Adrenaline would contain 1 g of epinephrine in 1,000 ml of solution. A 1:50 solution would contain 1g in 50 mL of solution. You can also express solute amounts using percentages. Percents indicate the amount of solute (in grams) in 100mL of liquid. For example: 3% saline means 3 g of NaCl in 100mL water 7.5% sodium bicarbonate would contain 7.5g of this chemical in 100mL of solution The examples given about (ratios and %) are ways of expressing the concentration of a solution. The properties and behavior of many solutions depend not only on the nature of the solute and solvent but also on the concentration of the solute in the solution. Chemists use many different units when expressing concentration; however, one of the most common units is molarity. Molarity (abbreviated as M) is the concentration of a solution expressed as the number of moles of solute per liter of solution: Molarity (M) = moles solute liters solution For example, a 0.25 M NaOH solution (this is read as 0.25 molar) contains 0.25 moles of sodium hydroxide in every liter of solution. Anytime you see the abbreviation M you should immediately think of it as mol/L. In order to calculate the molarity of a solution, you need to know two things: 1. the number of moles of solute 2. the total volume of the solution Once you know these two things – you can calculate M. But first you need to know what a mole is. The amount of a substance is often expressed in moles. Just like a dozen of something is equal to 12, a mole is equal to 6x1023 items. This is known as Avogadro’s number. So 2 moles is really 2 x (6x1023 items). To calculate molarity: Calculate the number of moles of solute present Calculate the number of liters of solution present Divide the number of moles of solute by the number of liters of solution. Example: What is the molarity of a solution if you dissolve 5.23 moles of a solute in 500 mL of solution? First, you need to realize that 500 mL of a solution is really 0.5 L. Once you convert your solution volume to liters – you are ready to plug it into the equation. Molarity = moles/volume (L) M = 5.23/0.5 M = 10.46 M This is pretty straightforward. But most of the time, you are not given the moles of the solute but have to calculate it first. To do this, you need to know the how much a mole weighs. The weight of a mole, for any element, is equal to the chemical’s weight in grams. We call this weight the molar mass. Molar mass is the grams of a chemical needed to make 1 mole. Molar mass is expressed as g/mole. We calculate molar mass using the periodic table of elements and the atomic mass for each element in the solute. Example: What is the molar mass of water? To do this, you need to know a few things: 1. the chemical formula for water = its H20 2. the atomic mass of hydrogen = its 1.007 or 1.0 3. the atomic mass of oxygen = its 15.999 or 16.0 So the atomic mass of H20 is about 18. Which means 1 mole of water would weight 18.0 g. So the molar mass of water is 18.0 g/mole. Let’s look at another problem. Example: What is the molarity of a solution prepared by dissolving 15.0 g of sodium hydroxide in 225 mL of solution? Step #1: Calculate the number of moles in 15.0 g of sodium hydroxide. The chemical formula for sodium hydroxide is NaOH. The molar mass for O is 16.0 g/mol The molar mass for H is 1.0 g/mol The molar mass for Na is 23.0 g/mol So the molar mass for NaOH is 40.0 g/mol. In other words, 1 mole of NaOH weighs 40 grams. But you only have 15.0 g. This means that 15.0 g is really 15/40 or 0.375 moles. Step #2: Convert your volume to liters Easy. You have 225 mL. This is 0.225 L Step #3: Calculate molarity Plug your data into the equation M = moles/volume (L) M = 0.375/0.225 M = 1.6666666 or 1.7M So dissolving 15.0 g of NaOH in 225 mL of water gives you a 1.7M solution. Worksheet problems Complete the following problems. Show your work and express your results using 2 significant digits. 1. What is the chemical weight of lithium chloride (LiCl)? 42.39g b. How much would 0.5 moles of LiCl weigh? 21.20g c. How much would 2 moles of LiCl weigh? 84.78g 2. Calculate the chemical weight/molar mass of ammonium chloride (NH4Cl) = 53.50g b. How much would 0.15 moles of NH4Cl weigh? 8.02g c. How much would 100 millimoles of NH4Cl weigh? 5.35g 3. Calculate the number of moles required to make 100 mL of a 3.0 M solution of CuSO4. Molar mass = 159.61g in one mole 3.0M = 478.83g in 1L of water In 100mL = 47.88g 4. A 4.0 g sugar cube (sucrose: C12H22O11) is dissolved in a 350 ml teacup filled with hot water. What is the molarity of the sugar solution? Molar mass = 342.30g in one mole 342.30 g in 1 L water = 1.0 M 4g in 1000mL water = 11.68mM 4g in 350mL water = 33.4mM 5. Calculate the molarity of the solution that results when 4.0 g of NaCl is dissolved in 30.0 ml of water. Molar mass = 58.54g in one mole 58.54g in 1000mL water = 1.0 M 58.54g in 30mL water = 33.33M 4g in 30mL water = 2.28M 6. How many grams of NaOH would you need to add to 0.35 liters of water to make a 1.50 M solution? Molar mass = 39.96g in one mole 39.96g in 1000mL water = 1.0 M 59.94g in 1000mL water = 1.5M 20.96g in 350mL water = 1.5M 7. A chemist dissolves 98.4 g of FeSO4 in enough water to make 2.0 L of solution. What is the molarity of the solution? Molar mass = 151.91g in one mole 151.91g in 1000mL water = 1.0 M 151.91g in 2000mL water = 0.5M 98.4g in 2000mL water = 0.32 M 8. How many moles of KBr are in 25.0 mL of a 1.23 M KBr solution? Molar mass = 119.00g in one mole 1.23M = 1.23 moles in 1000mL 1.23M = 0.03 moles in 25mL 9. Battery acid is generally 3M H2SO4. How many grams of H2SO4 are in 400 mL of this solution? Molar mass = 98.08g in one mole 98.08g in 1000mL water = 1.0 M 294.24g in 1000mL water = 3.0M 117.70g in 400mL water = 3.0M 10. How would you make 1.50 L of a 1.62 M NaCl solution? Molar mass = 58.54g in one mole 58.54g in 1000mL water = 1.0 M 94.83g in 1000mL water = 1.62M 142.24g in 1500mL water = 1.62M 11. Calculate the molarity of the solution that results when 5.0 ml of CH3OH (methyl alcohol or methanol) is dissolved in 5.0 ml of water. Molar mass of CH3OH = 32.04g in one mole 1mL weighs 0.792g 5mL weighs 3.96g 32.94g in 1000mL water = 1.0M 32.04g in 10mL water (5mL CH3OH + 5mL water) = 100M (decreasing the volume by 10-fold makes the Molarity 10-times more concentrated) 3.96g in 10mL water = 12.4M (3.96g is 8.1 times less than 32.04g; 100M/8.1 = 12.4M) 12. How would you make 2.0 Liters of 6.74 M CH3OH solution? Molar mass = 32.04g in one mole 32.04g in 1000mL water = 1.0 M 215.94g in 1000mL water = 6.74M 431.88g in 2000mL water = 6.74M 545.26mL up to 2000mL water = 6.74M 0.792g = 1mL 431.88 = 545.26mL of CH3OH required, add water until you reach 2000mL 13. 45.0 g of Ca(NO3)2 was used to create a 1.3 M solution. What is the volume of the solution? Molar mass of Ca(NO3)2 = 164.04g in one mole 164.04g in 1000mL = 1.0M 213.25g in 1000mL = 1.3M 45.0g is 4.74-times less than 213.25g So 1000mL/4.74 = 210.97mL In other words 45.0g in 210.97 or 211mL = 1.3M 14. How would you prepare 100mL of a 1:100 NaCl solution? Take 1mL of NaCl and add water up to 100mL 15. How would you prepare 10mL of a 1:20 glucose solution? 1mL of glucose + water up to 20mL = 1:20 0.5mL of glucose + water up to 10mL = 1:20 16. If a reagent bottle contains 10g of NaCl dissolved in 1L of water, what would its concentration be expressed as a ratio? What would it be as a percentage? Percentage = 10g in 1L is the same as 10g in 1000mL 10g in 1000mL is a 1% solution (same ratio as 1g in 100mL) Ratio = 1:100 17. How would you prepare 100mL of a 2% solution of LiCl? Dissolve 2g of LiCl solid in 100mL 18. How would you prepare 500ml of a 0.5% saline solution? Remember saline is really NaCl. To prepare 1% - take 1mL of saline and add water up to 100mL To prepare 0.5% - take 0.5mL and add water up to 100mL Or take 2.5mL and add water up to 500mL 19. How would you prepare 1L of a 10% calcium chloride solution? Take 100mL of a CaCl2 solution and add water to 1L Take 100g of CaCl2 solid and add water to 1L 20. If a reagent bottle contains 50g of glucose dissolved in 1L of water, what would its concentration be expressed as a percentage? 50g dissolved in 1000mL Is the same as: 5g dissolved in 100mL Which is another way of saying 5%