3M™ Tegaderm™ Absorbent Clear Acrylic Dressing Evaluation
Name of Evaluator
Title
Phone Number
Date
Health Care Facility Name
Department
Sales Representative
Phone Number
Do you presently use a hydrocolloid dressing?
Yes
No
If yes, which brand_________________________
If no, what absorbent dressing do you use?
Brand__________________ Shapes/Sizes_______________
Which Tegadern™ Absorbent Clear Acrylic Dressing did you try?
90800 oval 3 x 3.75 in
90801 oval 4.4 x 5.0 in
90802 square 5.9 x 6.0 in
90803 oval 5.6 x 6.25 in
90805 square 7.9 x 8.0 in
90807 sacral 4.7 x 5.7
Please check the body location where Tegaderm™ Absorbent Clear Acrylic Dressing was applied:
Coccyx/Sacrum
Shoulder
Elbow
Arm
Hip
Heel
Buttock
Other______________________
Please check the type of use:
Skin Tear
Cover dressing with wound filler
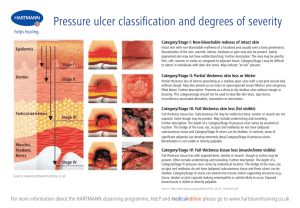
Stage II pressure ulcer Stage III pressure ulcer
Was a skin barrier used?
Yes
No
Donor Site
Other ________________
Brand_________________
Please rate Tegaderm™ Absorbent Clear Acrylic Dressing as it compares to dressing you use most often:
Much Better
Overall performance
Fluid handling capacity
Length of Wear
Nonadherency to wound bed
Bunching
Conformability
Condition of periwound skin
Adhesive residue on skin
Patient comfort
Ease of application
Ease of removal
Ability to monitor wound
Better
Same
Worse
Much Worse
Additional
Comments
Skin & Wound Care Division
3M Health Care
3M Center, Building 275-4W-02
St. Paul, MN 55144-1000
USA
1-800-228-3957
www.3M.com/healthcare
3M Canada
P.O. Box 5757
London, Ontario N6A 4T1
Canada
1-800-364-3577
www.3M.com/ca/healthcare
Please recycle.
Printed in U.S.A.
© 3M 2002. Portions Copyright 2006, 2010.
All rights reserved.
3M and Tegaderm are trademarks of 3M.
Used under license in Canada.
70-2009-6373-7
N/A