rcm7387-sup-0001-SI
advertisement

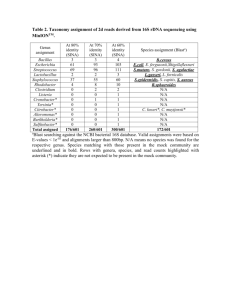
Supporting information Whole cells-based identification of electrochemically active bacteria in microbial fuel cells by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry Lijuan Zhang1, Yan Gao1, Linke Lai2 and Sam Fong Yau Li1,3* 1 Department of Chemistry, Faculty of Science, National University of Singapore, Singapore 117543, Singapore 2 NUS Graduate School for Integrative Sciences and Engineering, National University of Singapore, Singapore 119077, Singapore 3 NUS Environmental Research Institute, National University of Singapore, Singapore 117411, Singapore * Corresponding author. Tel.: +65 65162681; fax: +65 67791691. E-mail address: chmlifys@nus.edu.sg (S.F.Y. Li). Enrichment of EAB in MFCs Figure S1. SEM image of EAB enriched on the bio-anode of an MFC. Figure S2. (A) Mass spectra of unambiguously identified K. oxytoca (168 datacount) and K. pneumoniae (142 datacount), collected from aqueous anolyte, incubated on agar culture for 24 h at 37 ℃, extracted in 25% formic acid with vortex and ultra-sonication. (B) Mass spectrum producing conflicting result (indicating both K. oxytoca and K. pneumoniae, 516 datacount), collected from attached biofilm, extracted in 25% formic acid with vortex and ultra-sonication. Effects of formic acid and ultrasonic wave on in-situ protein extraction Figure S3. Datacount acquired before and after protein extraction by 25% formic acid with and without ultra-sonication, incubated at 37 ℃ for 24 h. Figure S4. Spectra of identified species C. testosteroni (99.90% confidence) extracted with deionized water, 25% FA and 25% FA with sonication, incubated at 37oC for 24 h. Figure S5. Polarization and power curves for MFC utilized for the enrichment of EAB. EAB was incubated in 50 mM phosphate-buffered saline (pH = 7.00) with an initial D(+)-Glucose concentration of 1 g L-1. Phylogenetic analysis of EAB by 16S ribosomal RNA sequencing Protocol for 16S Bacteria Species Barcoding Genomic DNA extraction was done using commercial DNA extraction kit via beads bashing method. Purified PCR product was cloned into pJET1.2/blunt vector using commercial cloning kit according to the manufacturer’s protocol. Positive clones were sent for sequencing, and sequence results were then analyzed and BLAST in 16S ribosomal RNA sequences (Bacteria only) Database excluding uncultured bacteria bacterium (taxid:77133). Phylogeny tree was constructed using NCBI Blast Tree method. NOTE: gDNA was used as template in PCR to amplify 16S rRNA genes, not RNA itself. The gene (fragment of DNA) amplified encodes for the protein rRNA. Figure S6. Phylogenetic tree of 16S rRNA gene (partial sequences) of EAB in MFCs. The tree was calculated by the Neighbor-Joining method algorithm and a 50% conservation filter. The scale bar denotes 5% sequence divergence, and the numbers at the nodes represent bootstrap values (1000 replicates). Group A: Group 1. Genus Clostridium (C3), Group 2. Genus Acetoanaerobium (C1, C29, C40) and Group 3. Genus Fusibacter (C28); Group B: Group 4. Family Enterobacteriaceae (C26, C27, the genus Klebsiella, Raoultella, Plantoea could not be determined) and Group 5. Genus Stenotrophomonas (C30, not Pseudomonas).