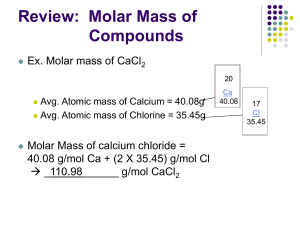
Chemistry (K. Stephens)
advertisement
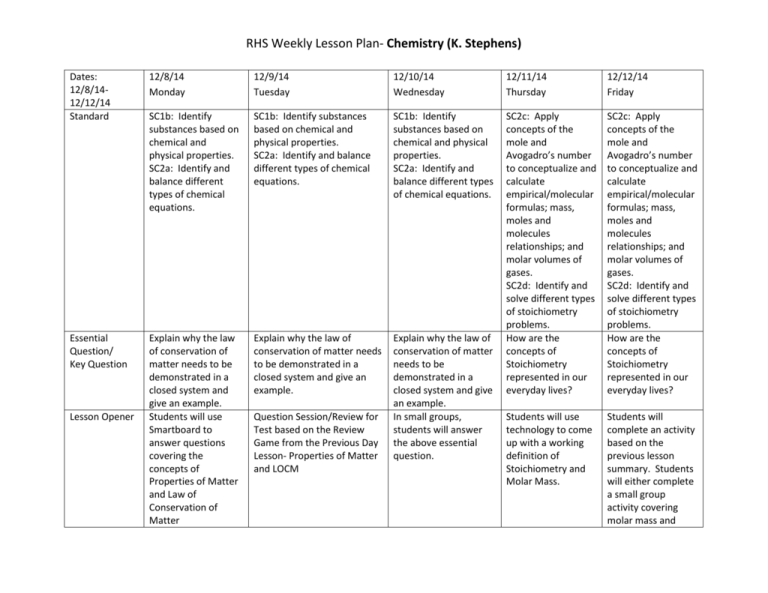
RHS Weekly Lesson Plan- Chemistry (K. Stephens) Dates: 12/8/1412/12/14 Standard 12/8/14 Monday 12/9/14 Tuesday 12/10/14 Wednesday 12/11/14 Thursday 12/12/14 Friday SC1b: Identify substances based on chemical and physical properties. SC2a: Identify and balance different types of chemical equations. SC1b: Identify substances based on chemical and physical properties. SC2a: Identify and balance different types of chemical equations. SC1b: Identify substances based on chemical and physical properties. SC2a: Identify and balance different types of chemical equations. Essential Question/ Key Question Explain why the law of conservation of matter needs to be demonstrated in a closed system and give an example. Students will use Smartboard to answer questions covering the concepts of Properties of Matter and Law of Conservation of Matter Explain why the law of conservation of matter needs to be demonstrated in a closed system and give an example. Explain why the law of conservation of matter needs to be demonstrated in a closed system and give an example. In small groups, students will answer the above essential question. SC2c: Apply concepts of the mole and Avogadro’s number to conceptualize and calculate empirical/molecular formulas; mass, moles and molecules relationships; and molar volumes of gases. SC2d: Identify and solve different types of stoichiometry problems. How are the concepts of Stoichiometry represented in our everyday lives? SC2c: Apply concepts of the mole and Avogadro’s number to conceptualize and calculate empirical/molecular formulas; mass, moles and molecules relationships; and molar volumes of gases. SC2d: Identify and solve different types of stoichiometry problems. How are the concepts of Stoichiometry represented in our everyday lives? Students will use technology to come up with a working definition of Stoichiometry and Molar Mass. Students will complete an activity based on the previous lesson summary. Students will either complete a small group activity covering molar mass and Lesson Opener Question Session/Review for Test based on the Review Game from the Previous Day Lesson- Properties of Matter and LOCM RHS Weekly Lesson Plan- Chemistry (K. Stephens) Procedures/Str ategies Finish Review SheetProperties of Matter Test- Properties of Matter and Law of Conservation of Matter Finish Lab from Previous WeekProperties of Matter Review Game for Test- Properties of Matter Lesson Summary Based on the Review Game for Test, a brief review session will occur covering specific topics on Properties of Matter and LOCM. DiscussionStoichiometry, Moles, Molar Mass Examples- Mole Ratios, Molar Masses, and Mole to Mole Stoichiometry Problems. Finish Test- Properties of Matter and Law of Conservation of Matter Students will use Kahoots to review the concepts of Properties of Matter and LOCM. Group Activity- Mole to Mole Stoichiometry Problems. Students will answer a question covering Mole to Mole Stoichiometry using Kahoots. mole to mole stoichiometry or small group discussions will occur covering mole to mole stoichiometry. Notes/ExamplesMoles to Mass Stoichiometry Problems Station ActivityMole to Mole and Moles to Mass Stoichiometry Problems Socrative will be used to answer questions covering Molar Mass, Mole to Mole Stoichiometry, and Moles to Mass Stoichiometry RHS Weekly Lesson Plan- Chemistry (K. Stephens)