Additional Results DNA sequences of plasmids
advertisement

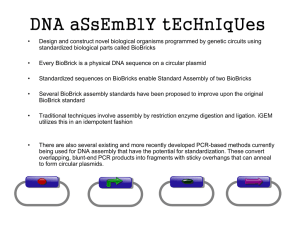
Supplementary Table 1 - Transformants of T. reesei RUT-C30 constructed in this study. Supplementary Table 2 – Sequences of oligos. Phosphinothricin purification Transformation of T. reesei Immobilized metal-ion affinity chromatography RNA preparation and quantitative real-time reverse transcription PCR Plasmid construction Supplementary Figure 1. Restriction enzyme map of pPK2, pPK1s and two based vectors pSB902 and pSB903. Supplementary Figure 2. The transformation efficiency and relative hpt mRNA expression level using five short promoters. Supplementary Figure 3. Alkaline endoglucanase activities in s-pSB902-V3 transformants and RUT-C30 with no additional plasmids. Supplementary Figure 4. Copy number of the deletion cassette in two Δcbh1 transformants (CBH1-rgf.1 and CBH1-rgf.7). Additional Results: DNA sequences of plasmids Additional References Supplementary Table 1. Transformants of T. reesei RUT-C30 constructed in this study. Strain Relevant features promoter reporter gene Source RUT-C30 parent strain - - ATCC pBar RUT-C30 harboring vector pBar As. nidulans trpC bar This study p9B RUT-C30 harboring vector p9B truncated xyn2 bar This study C1-s RUT-C30 harboring vector C1-s As. nidulans gpd hpt This study C1-pAs RUT-C30 harboring vector C1-pAs As. Nidulans amdS hpt This study C1-pbNs RUT-C30 harboring vector C1-pbNs As. nidulans trpC hpt This study C1-p7s RUT-C30 harboring vector C1-p7s xyn1 hpt This study C1-p9s RUT-C30 harboring vector C1-p9s truncated xyn2 hpt This study s-pSB902-V3 RUT-C30 harboring vector s-pSB902-V3 truncated xyn2 egv3 This study bns-pSB101-rfp RUT-C30 harboring vector bns-pSB101-rfp As. nidulans trpC, cbh1 hpt, rfp This study CBH1-rgf.1, CBH1-rgf.7 pG01-rfp transformant cbh1 This study rfp RUT-C30::pG01-rfp--gfp RUT-C30::pG01-rfp harboring vector 9B-pSB401-gfp cbh1, cbh2 rfp, gfp This study CBH1-V3.1 pG01-V3 transformant cbh1 egv3 This study CBH2-H2.1 pG02-H2 transformant cbh2 H. cbh2 This study CBH1-V3--CBH2-H2.1 pG01-V3 and pG02-H2 transformant cbh1, cbh2 egv3, H. cbh2 This study Supplementary Table -2. Sequences of oligos. Name oligos Sequences (5’ to 3’) bar-forward cgaaCACGTG(PmlI)ATGAGCCCAGAACGACGCC bar-reverse cgaaCGCGCGCG(MauBI)TCAGATCTCGGTGACGGGCA PPT-forward cgaaTTAATTAA(PacI)GTTAACTCTAGA(XbaI)gatatcGTCGGAGACAGAAGATGATATT PPT-reverse cgaaATTTAAAT(SwaI)GAGCTCACTAGT(SpeI)TTTTTTTTAATTTGTTGGAGATTTCA VF cgaa CACGTG(PmlI)GCCGACGGCCGCAGCAC VR cgaa CGCGCGCG(MauBI)TTACAGGCACTGGTGGTACCAG C15F cgaaTTAATTAA(PacI)GATATCAGTATGTAAGTCCAA C15R cgaaACTAGT(SpeI)TGCTACTAGACACTGCTATCGG C13F cgaaTCTAGA(XbaI)CGTGGCGAAAGCCTGACG C13R cgaaATTTAAAT(SwaI)ATCAATTGCTGCTTCATACTA C25F cgaaTTAATTAA(PacI)CTAGTCAATGGTAGCAGATCAGTC C25R cgaaACTAGT(SpeI)GAGATATAAGGCAGAATGGATACGA C23F cgaaTCTAGA(XbaI)TAGATTCCAATTACTCCACCTCTTG C23R cgaaATTTAAAT(SwaI)ACTACGCTAATCGGATAGATATTCG qsar-f TGGATCGTCAACTGGTTCTACGA qsar-r GCATGTGTAGCAACGTGGTCTTT qhpt-f AGCGAGAGCCTGACCTATTGC qhpt-r CCATGTAGTGTATTGACCGATTCCTT Phosphinothricin purification Phosphinothricin (PPT, also called glufosinate), the active ingredient in basta, is currently expensive in its pure form. Basta (Bayer Cropscience, Australia), an herbicide with PPT as an active ingredient (~18%), was used in the present study. Because PPT is highly soluble in water, a simple extraction separates it from the pigment and other non-specific inhibitory ingredients. In the current experiment, Basta was extracted three times with an equal volume of 1-butanol, the solution was lyophilized and the resulting gel was then dissolved in water (final concentration, 200 mg/mL). Transformation of T. reesei Transformation of the hpt marker vectors was performed based on protocols described by Covert et al. (2001). The PPT marker vectors pBar, p9B, 9B-pSB401-H2 and 9B-pSB401-gfp were introduced into RUT-C30 by Agrobacterium-mediated transformation (Moon et al. 2008) with the following modifications. The transformant was selected by transferring each filter paper onto plates containing M-100 medium amended with 10 g glucose l-1 and 400 μg purified PPT ml-1. The medium also contained 100 μg of cefotaxime (Generay, Shanghai, China) ml-1 to kill bacteria. After one day of incubation at 27°C, the filter paper was overlaid with 10 ml freshly prepared M-100 agar containing 300 μg purified PPT ml-1. Plates were incubated for 4 to 7 days at 27°C until the transformants had grown to the surface of the agar. Each primary transformant was transferred to a single well of a 24-well microtiter plate containing M-100 medium (300 μg purified PPT ml-1). Immobilized metal-ion affinity chromatography Fermentation broth culture supernatant (9 ml; centrifuged at 7,000 g for 10 min at 4°C) was collected for chromatography by adding 1 ml 10× NPI-10 (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole) and adjusting the pH to 8.0 using NaOH. Mycelia were harvested by filtration and then washed twice with distilled water. Excess moisture was removed by pressing the mycelia between sheets of Whatman 3mm filter paper (Whatman), after which the mycelia were immediately frozen in liquid nitrogen and ground into a fine powder. The powder was suspended in an appropriate volume of NPI-10 and the pH was adjusted to 8.0 using NaOH. Nitrilotriacetic acid (Ni-NTA) chromatography was carried out according to the Ni-NTA Superflow Cartridge Handbook (www.qiagen.com). All chemicals were purchased from Merck, except for Ni-NTA-Superflow (Qiagen). RNA preparation and quantitative real-time reverse transcription PCR About 100 mg of T. reesei mycelium was harvested. Total RNA was extracted using the FastRNA Pro Red Kit (MPbio, U. S. A.), according to the manufacturer’s instructions. Reverse transcription was performed with 1000 ng of total RNA using TransScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (TransGen, China), according to the manufacturer’s instructions. For qRT-PCR, the TransStart TipTop Green qPCR SuperMix (TransGen, China) was used with 200 nM of forward and reverse primers (see Supplemental Material Supplementary Table -2) and 1 μl of 10-fold diluted cDNA in a final volume of 20 μl. The conditions of qPCR: 30s at 94oC, 45 cycles of 5s at 94 oC and 30s at 60 oC,followed by dissociation stage. For hpt transcription analysis, a SYBR green assay with reference to the small GTPase gene (sar1) was performed (Steiger et al. 2010). Thermocycling was performed in an ABI StepOne Plus thermocycler (Applied Biosystems, U. S. A.). Plasmid construction RUT-C30 genomic DNA was prepared according to the method described by Penttilä et al. (1987). The expression vector pWEF31-V (Zhang et al. 2014) was used as the source of the alkaline endoglucanase, egv. The template for the 0.64-kb Aspergillus nidulans amdS promoter was the plasmid pMS-HALS (Steiger et al. 2011). The template for the 0.56-kb bar, 1.0-kb PPT-resistant box and 0.36-kb As. nidulans trpC promoter was the plasmid pTJK1 (Jones et al. 2007). The template for the 1.02-kb hpt was the plasmid pPK1s (Zhang et al. 2014). The vectors were built by exploiting the compatibility of cohesive ends generated by XbaI and SpeI in pPK2-derived BioBrick base vector, as previously described (Covert et al. 2001; Wang et al. 2014). All primers used in this section are listed in Supplemental Material Supplementary Table -2. Expression vectors pSB902 and pSB903: The expression vectors pSB902 and pSB903 (Fig. S1) (Supplemental Data File) were constructed using the truncated xyn2 promoter (0.24-kb), with or without native signal peptide, respectively, and the nos terminator (GenBank: KF499077.1). pSBa01 and pSBbN (Supplemental Data File) were constructed using the amdS or trpC promoter, respectively, and the nos terminator. The gene expression device pSB701 (Supplemental Data File) was constructed with the core promoter region of the gene xyn1 (Martinez et al. 2008) and the nos terminator. Hygromycin B resistance vectors pAs, pbNs, p7s and p9s: The 1.02-kb hpt was subcloned into the PmlI/MauBI site of pSBa01, pSBbN, pSB701 and pSB903 to generate the hygromycin B resistance vectors pAs, pbNs, p7s and p9s, respectively (Fig. 1). PPT resistance vectors pBar and p9B: The PPT-resistant box was amplified with the PPT-forward/PPT-reverse primers. The 1.0-kb PPT-resistant box was subcloned into the PacI/SpeI sites of pPK1s to produce the PPT resistance vector pBar. The 0.56-kb bar gene was amplified with the bar-forward/bar-reverse primers, then subcloned into the PmlI/MauBI sites of pSB903 to develop the PPT resistance vector p9B (Fig. 1). Alkaline endoglucanase EGV expression vector s-pSB902-V3: PCR amplification of egv was carried out using the VF/VR primers. The 0.87-kb egv gene was subcloned into the PmlI/MauBI sites of pSB902 to generate pSB902-V3. The 2.7-kb hygromycin-resistant part (the smaller fragment from pPK1s digested with PacI/SpeI) was subcloned into the PacI/XbaI site of the precursor vector to generate s-pSB902-V3 (Fig. 1). Alkaline endoglucanase EGV expression vector bns-pSB101-V3: The 1.7-kb hygromycin-resistant part (the smaller fragment from pbNs digested with PacI/SpeI) was subcloned into the PacI/XbaI site of pSB101-V3 to generate bns-pSB101-V3 (Fig. 1). Alkaline cellobiohydrolase Humicola CBH2 expression vector 9B-pSB401-H2: The 1.1-kb PPT-resistant part (the smaller fragment from p9B digested with PacI/SpeI) was subcloned into the PacI/XbaI site of pSB401-H2 to generate 9B-pSB401-H2 (Fig. 1). cbh1 knockout vectors C1-s, C1-pAs, C1-pbNs, C1-p7s and C1-p9s: In addition, 1.8 kb of the upstream non-coding region and 2.1 kb of the downstream non-coding region from T. reesei cbh1 were amplified using the primer pairs C15F/R and C13F/R, respectively. PCR fragments were digested with PacI/SpeI or XbaI/SwaI, respectively, and ligated into the compatible sites (pPK1s, pAs, pbNs, p7s and p9s) to generate C1-s, C1-pAs, C1-pbNs, C1-p7s and C1-p9s (Fig. 1). Reporter gene expression vectors 9B-pSB401-gfp and bns-pSB101-rfp: The green fluorescent protein expression vector pSB401-gfp (Wang et al. 2014) was digested using XbaI/SwaI. The 1.9-kb DNA fragment was purified and subcloned into the SpeI/SwaI sites of p9B to generate 9B-pSB401-gfp. The red fluorescent protein expression vector pSB101-rfp (Wang et al. 2014) was digested with XbaI/SwaI, and the 1.9-kb DNA fragment was purified and subcloned into the SpeI/SwaI sites in pbNs to generate bns-pSB101-rfp. Versatile vectors pG01-rfp and pG01-V3 for heterologous gene expression and knockout of cbh1: In addition, 1.8 kb of the upstream non-coding region and 2.1 kb of the downstream non-coding region from T. reesei cbh1 were ligated into bns-pSB101-gfp and bns-pSB101-V to generate pG01-rfp and pG01-V3 (Fig. 1), respectively. A versatile vector pG02-H2 for heterologous gene expression and knockout of cbh2: Finally, 1.8 kb of the upstream non-coding region and 2.1 kb of the downstream non-coding region from T. reesei cbh2 were amplified using the primer pairs C25F/R and C23F/R, respectively. PCR fragments were digested with PacI/SpeI or XbaI/SwaI, respectively, and ligated into 9B-pSB401-H2 to generate pG02-H2 (Fig. 1). Supplementary Figure 1. Restriction enzyme map of pPK2 (Covert et al. 2001), ppk1s (Zhang et al. 2014) and two based vectors pSB902 and pSB903. Transformation efficiency Ralative expression level of hpt 100 8 6 60 4 40 X fold expression Transformation efficiency 80 2 20 0 0 C1-s C1-pAs C1-pbNs C1-p7s C1-p9s strains Supplementary Figure 2. The transformation efficiency and relative hpt mRNA expression level using five short promoters. Alkaline endoglucanase activity (IU/mL) 3.0 s-pSB902-V3 transformant RUT-C30 2.5 2.0 1.5 1.0 0.5 0.0 xylan xylose lactose glucose Carbon source Supplementary Figure 3. Alkaline endoglucanase activities in s-pSB902-V3 transformants and RUT-C30 with no additional plasmids. After cultivation with four different carbon sources: xylan, xylose, lactose and glucose, alkaline endoglucanase activity was determined in 50 mM sodium phosphate buffer, pH 9.0 at 50°C. Error bars represent the mean ± SEM (n= 3 samples) from the same experiment. Supplementary Figure 4. Copy number of the deletion cassette in two Δcbh1 transformants (CBH1-rgf.1 and CBH1-rgf.7). Additional Results DNA sequences of plasmids >pSB902 TTAATTAAGTTAACTCTAGAgatatcAACAACTACTAGACTGGGTAAATTGGTC AATGGCCAGCCGCTCGGCCGTGCGGAGACGAGGCAAGCTTGATGAGGCCA AATTATCCGTCAACTGTCTTATAAAGGAGCCCATGCCAAACCCCCCCTAAA GACTCAAGAAGCCAAACCTGAACAACCCCAGCACCTGAACAGTCATACAA CCCCTCCAAGCCCAAAAGACACAACAACTCCTACTAGCTGAAGCAAGAAG ACATCAACATGGTCTCCTTCACCTCCCTCCTCGCCGGCGTCGCCGCCATCTC GGGCGTCTTGGCCGCTCCCGCCGCCGAGGTCGAATCCGTGcacgtgAAAcgcgc gcgCcaccaccaccaccaccactaatagGATCGTTCAAACATTTGGCAATAAAGTTTCTTA AGATTGAATCCTGTTGCCGGTCTTGCGATGATTATCATATAATTTCTGTTGAA TTACGTTAAGCATGTAATAATTAACATGTAATGCATGACGTTATTTATGAGAT GGGTTTTTATGATTAGAGTCCCGCAATTATACATTTAATACGCGATAGAAAAC AAAATATAGCGCGCAAACTAGGATAAATTATCGCGCGCCGTGTCATCTATGT TACTAGATCACTAGTGAGCTCATTTAAATAAGCTT >pSB903 TTAATTAAGTTAACTCTAGAgatatcACAACTACTAGACTGGGTAAATTGGTCA ATGGCCAGCCGCTCGGCCGTGCGGAGACGAGGCAAGCTTGATGAGGCCAA ATTATCCGTCAACTGTCTTATAAAGGAGCCCATGCCAAACCCCCCCTAAAG ACTCAAGAAGCCAAACCTGAACAACCCCAGCACCTGAACAGTCATACAAC CCCTCCAAGCCCAAAAGACACAACAACTCCTACTAGCTGAAGCAAGAAGA CATCAACATGGTCcacgtgAAAcgcgcgcgCcaccaccaccaccaccactaatagGATCGTTCA AACATTTGGCAATAAAGTTTCTTAAGATTGAATCCTGTTGCCGGTCTTGCGA TGATTATCATATAATTTCTGTTGAATTACGTTAAGCATGTAATAATTAACATGT AATGCATGACGTTATTTATGAGATGGGTTTTTATGATTAGAGTCCCGCAATTA TACATTTAATACGCGATAGAAAACAAAATATAGCGCGCAAACTAGGATAAAT TATCGCGCGCCGTGTCATCTATGTTACTAGATCACTAGTGAGCTCATTTAAAT AAGCTT >pSBa01 TTAATTAAGTTAACTCTAGAgatatcTCTACGCCAGGACCGAGCAAGCCCAGAT GAGAACCGACGCAGATTTCCTTGGCACCTGTTGCTTCAGCTGAATCCTGGC AATACGAGATACCTGCTTTGAATATTTTGAATAGCTCGCCCGCTGGAGAGCA TCCTGAAtgcaagtaacaaccgtagaggctgacacggcaggtgttgctagggagcgtcgtgttctacaaggccaga cgtcttcgcggttgatatatatgtatgtttgactgcaggctgctcagcgacgacagtcaagttcgccctcgctgcttgtgcaat aatcgcagtggggaagccacaccgtgactcccatctttcagtaaagctctgttggtgtttatcagcaatacacgtaatttaaac tcgttagcatggggctgatagcttaattaccgtttaccagtgccgcggttctgcagctttccttggcccgtaaaattcggcgaa gccagccaatcaccagctaggcaccagctaaaccctataattagtctcttatcaacaccatccgctcccccgggatcaatga ggagaatgagggggatgcggggctaaagaagcctacataaccctcatgccaactcccagtttacactcgtcgagccaaca tcctgactataagctaacacagaatgGCCcacgtgAAAcgcgcgcgCcaccaccaccaccaccactaatagGAT CGTTCAAACATTTGGCAATAAAGTTTCTTAAGATTGAATCCTGTTGCCGGTC TTGCGATGATTATCATATAATTTCTGTTGAATTACGTTAAGCATGTAATAATTA ACATGTAATGCATGACGTTATTTATGAGATGGGTTTTTATGATTAGAGTCCCG CAATTATACATTTAATACGCGATAGAAAACAAAATATAGCGCGCAAACTAGG ATAAATTATCGCGCGCCGTGTCATCTATGTTACTAGATCACTAGTGAGCTCAT TTAAATAAGCTT >pSBbN TTAATTAAGTTAACTCTAGAgatgtgTGATATTGAAGGAGCACTTTTTGGGCTT GGCTGGAGCTAGTGGAGGTCAACAATGAATGCCTATTTTGGTTTAGTCGTC CAGGCGGTGAGCACAAAATTTGTGTCGTTTGACAAGATGGTTCATTTAGGC AACTGGTCAGATCAGCCCCACTTGTAGCAGTAGCGGCGGCGCTCGAAGTG TGACTCTTATTAGCAGACAGGAACGAGGACATTATTATCATCTGCTGCTTGG TGCACGATAACTTGGTGCGTTTGTCAAGCAAGGTAAGTGAACGACCCGGT CATACCTTCTTAAGTTCGCCCTTCCTCCCTTTATTTCAGATTCAATCTGACTT ACCTATTCTACCCAAGCCTCGATCATGGCAcacgtgAAAcgcgcgcgCcaccaccaccac caccactaatagGATCGTTCAAACATTTGGCAATAAAGTTTCTTAAGATTGAATCC TGTTGCCGGTCTTGCGATGATTATCATATAATTTCTGTTGAATTACGTTAAGC ATGTAATAATTAACATGTAATGCATGACGTTATTTATGAGATGGGTTTTTATGA TTAGAGTCCCGCAATTATACATTTAATACGCGATAGAAAACAAAATATAGCG CGCAAACTAGGATAAATTATCGCGCGCCGTGTCATCTATGTTACTAGATCAC TAGTGAGCTCATTTAAATAAGCTT >pSB701 TTAATTAAGTTAACTCTAGAgatatcAAGCAACTACGTAAAACTCCATGAGATT GCAGATGCGGCCCACTGGAATACAACATCCTCCGCAAGTCCGACATGAAGC CCCTTGACTTGATTGGCAGGCTAAATGCGACATCTTAGCCGGATGCACCCC AGATCTGGGGAACGCGCCGCTTGAGGCCCGAAGCGCCGGGTTCGATGCAT TACTGCCATATTTCAGCAGTTAACTAGGACCGGCTTGTGTCGATATTGCGGG TGGCGTTCAATCTATTCCGGCACTCCTATGCCGTTTGATCCGATACCTGGAG GGCGTGCTTTAGGCAAAATGCCAAGCTTCGAGGATACTGTACGAGCCGCTT TCAACCTCACTTGATGATGTCTGAGTTTCATCAAGAGAATTGAAGTCAAAG CTCAAATCATGATGTGAAGAGGTTTTGAATGTGGAAGAATTCTGCATATATA AAGCCATGGAAGAAGACGTAAAACTGAGACAGCAAGCTCAACTGCATAGT ATCGACTTCAAGGAAAACACGCACAAATAATCATCATGGCCcacgtgAAAcgcg cgcgCcaccaccaccaccaccactaatagGATCGTTCAAACATTTGGCAATAAAGTTTCTT AAGATTGAATCCTGTTGCCGGTCTTGCGATGATTATCATATAATTTCTGTTGA ATTACGTTAAGCATGTAATAATTAACATGTAATGCATGACGTTATTTATGAGA TGGGTTTTTATGATTAGAGTCCCGCAATTATACATTTAATACGCGATAGAAAA CAAAATATAGCGCGCAAACTAGGATAAATTATCGCGCGCCGTGTCATCTATG TTACTAGATCACTAGTGAGCTCATTTAAATAAGCTT References Covert SF, Kapoor P, Lee M-h, Briley A, Nairn CJ (2001) Agrobacterium tumefaciens-mediated transformation of Fusarium circinatum. Mycol Res 105: 259-264 Jones CA, Greer-Phillips SE, Borkovich KA (2007) The response regulator RRG-1 functions upstream of a mitogen-activated protein kinase pathway impacting asexual development, female fertility, osmotic stress, and fungicide resistance in Neurospora crassa. Mol Biol Cell 18: 2123-2136 Moon YS, Donzelli BG, Krasnoff SB, McLane H, Griggs MH, Cooke P, Vandenberg JD, Gibson DM, Churchill AC (2008) Agrobacterium-mediated disruption of a nonribosomal peptide synthetase gene in the invertebrate pathogen Metarhizium anisopliae reveals a peptide spore factor. Appl Environ Microbiol 74: 4366-4380 Penttilä M, Nevalainen H, Rättö M, Salminen E, Knowles J (1987) A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 61: 155-164 Steiger MG, Mach RL, Mach-Aigner AR (2010) An accurate normalization strategy for RT-qPCR in Hypocrea jecorina (Trichoderma reesei). J Biotechnol 145: 30-37 Steiger MG, Vitikainen M, Uskonen P, Brunner K, Adam G, Pakula T, Penttilä M, Saloheimo M, Mach RL, Mach-Aigner AR (2011) Transformation system for Hypocrea jecorina (Trichoderma reesei) that favors homologous integration and employs reusable bidirectionally selectable markers. Appl Environ Microbiol 77: 114-121 Wang W, Meng F, Liu P, Yang S, Wei D (2014) Construction of a promoter collection for genes co-expression in filamentous fungus Trichoderma reesei. J Ind Microbiol Biotechnol 41: 1709-1718 Zhang G, Zhu Y, Wei D, Wang W (2014) Enhanced Production of Heterologous Proteins by the Filamentous Fungus Trichoderma reesei via Disruption of the Alkaline Serine Protease SPW Combined with a pH Control Strategy. Plasmid 71: 16-22