Lab Report 1 - obelkemalvatansever
advertisement

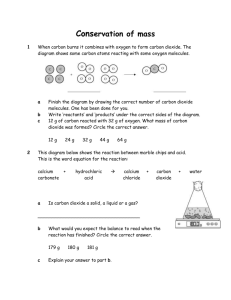
İhsan Doğramacı Foundation Bilkent Erzurum Laboratory School 2011-2012 Academic Year Spring Semester Chemistry Lab Report-1 Acids and Bases in the Kitchen 06.04.2012 Kemal Vatansever 9-D 216 Purpose 1. The purpose of this lab activity was to see the reaction of chemicals (acids and bases) in the kitchen and the products of this reaction. 2. The purpose of the second part of lab activity was to see that the balloon didn’t burst when a skewer goes through it. 3. The purpose of third part of this lab activity was to see the effect of carbon dioxide on the fire’s burning process. Hypothesis 1. When the acidic substances in the kitchen react with carbonates, most of the time a salt, water, and carbon dioxide is formed. 2. When liquid detergent covers the entering and the exit of the skewer, there will be less amount of gas outlet that affects the pressures’ of inside and outside the balloon. 3. When the carbon dioxide gas is poured on the burning candle, the heavier carbon dioxide will precipitate in the air so the fire will be burn out. Equipment Plastic Bottle Skewer Candle Balloon Liquid Detergent Procedure At the beginning of the experiment, vinegar was dropped into the plastic bottle that contains 5% solution of 𝐶𝐻3 𝐶𝑂𝑂𝐻 (Acetic Acid). After that, containing𝑁𝑎𝐻𝐶𝑂3, baking soda which is carbonate, added into the vinegar. And immediately, the balloon was scarfed to the rim of the bottle to not lose any products of the reaction between to matters. Then bubbles occurred on this mixture of two matters and the balloon swelled, so that carbon dioxide was occurred in this reaction. Also, the liquid’s color has changed. Thus water occurred in the reaction. At the bottom of the bottle, there was an insoluble salt that precipitated which is Sodium Acetate (𝐶2 𝐻3 𝑁𝑎𝑂2 ). This reaction was neutralization reaction that gives an insoluble salt, water, and carbon dioxide as a product. Acetic Acid +Sodium Bicarbonate Sodium Acetate + Water + Carbon dioxide 𝐶𝐻3 𝐶𝑂𝑂𝐻 +𝑁𝑎𝐻𝐶𝑂3 𝐶2 𝐻3 𝑁𝑎𝑂2 + 𝐻2 𝑂 + 𝐶𝑂2 After that, the balloons that are full of carbon dioxide were knotted to test with liquid detergent. The liquid detergent was smeared to the both front and back surface of it. When the skewer is stabbed trough the balloon, the balloon didn’t explode because the detergent prevented carbon dioxide from escaping immediately to the low pressure. Lastly, the carbon dioxide remaining in the bottle is dropped on to the burning candle. The candle burnt out because the heavier particles of carbon dioxide in the air filled the sink on the candle’s rope and prevented the requirements of the flame: Oxygen. Thus the fire burnt out. Conclusion This experiment confirms the hypothesis that the acidic substances react with carbonates, most of the time a salt, water, and carbon dioxide is formed. This experiment confirms the hypothesis that there will be less amount of gas outlet that affects the pressures’ of inside and outside the balloon when liquid detergent covers the entering and the exit of the skewer. This experiment confirms the hypothesis that the heavier carbon dioxide will precipitate in the air so the fire will be burn out when the carbon dioxide gas is poured on the burning candle. Lab Rules and Safety in Lab 1. Conduct yourself in a responsible manner at all times in the laboratory. 2. Never work alone in the laboratory. No student may work in the science classroom without the presence of the teacher. 3. When first entering a science room, do not touch any equipment, chemicals, or other materials in the laboratory area until you are instructed to do so. 4. Perform only those experiments authorized by your teacher. Carefully follow all instructions, both written and oral. 5. Do not eat food, drink beverages, or chew gum in the laboratory. Do not use laboratory glassware as containers for food or beverages. 6. Be prepared for your work in the laboratory. Read all procedures thoroughly before entering the laboratory. Never fool around in the laboratory. 7. Always work in a well-ventilated area. 8. Observe good housekeeping practices. Work areas should be kept clean and tidy at all times. 9. Dispose of all chemical waste properly. Never mix chemicals in sink drains. Sinks are to be used only for water. Check with your teacher for disposal of chemicals and solutions. 10. Labels and equipment instructions must be read carefully before use. Set up and use the equipment as directed by your teacher. 11. Keep hands away from face, eyes, mouth, and body while using chemicals or lab equipment. Wash your hands with soap and water after performing all experiments. 12. Experiments must be personally monitored at all times. Do not wander around the room, distract other students, startle other students or interfere with the laboratory experiments of others. 13. Know the locations and operating procedures of all safety equipment including: first aid kit(s), and fire extinguisher. Know where the fire alarm and the exits are located. 14. Know what to do if there is a fire drill during a laboratory period; containers must be closed, and any electrical equipment turned off. 15. Any time chemicals, heat, or glassware are used, students will wear safety goggles. 16. Dress properly during a laboratory activity. Long hair, dangling jewelry, and loose or baggy clothing are a hazard in the laboratory. Long hair must be tied back, and dangling jewelry and baggy clothing must be secured. Shoes must completely cover the foot. 17. A lab coat or smock should be worn during laboratory experiments. 18. Report any accident or injury to the teacher immediately, no matter how trivial it seems. 19. If you or your lab partner is hurt, immediately call the teacher's name to get the teacher's attention. 20. If a chemical should splash in your eye(s) or on your skin, immediately flush with running water for at least 20 minutes. 21. All chemicals in the laboratory are to be considered dangerous. Avoid handling chemicals with fingers. Always use tweezers. When making an observation, keep at least 1 foot away from the specimen. Do not taste, or smell any chemicals. 22. Check the label on all chemical bottles twice before removing any of the contents. Take only as much chemical as you need. 23. Never remove chemicals or other materials from the laboratory area. 24. Never handle broken glass with your bare hands. Use a brush and dustpan to clean up broken glass. Place broken glass in the designated glass disposal container.