Measurement of Hardness of water The hardness of water is mainly

Measurement of Hardness of water
The hardness of water is mainly due to the presence of carbonates, bi-carbonates, chlorides and sulphates of calcium and magnesium in dissolved form. These salts cause excessive consumption of soap used for cleaning purpose.
The formation of solid calcium carbonate is an endothermic process . Thus, when water containing both carbonate and calcium ions is heated, calcium carbonate can precipitate out onto the walls of pipes, boilers, and household items such as tea pots.
This can shorten the life-time of some of these items.
The World Health Organization ( WHO) says that "there does not appear to be any convincing evidence that water hardness causes adverse health effects in humans.
Total Hardness is composed of two components, temporary and permanen t hardness.
The temporary hardness is due to the presence of carbonates and bi-carbonates of calcium and magnesium. It can be easily removed by boiling the water or by adding lime to water.
Both calcium bi-carbonate and magnesium bi-carbonate decompose when heated. The original insoluble carbonate is reformed. This happens when water is boiled. The precipitation reactions are as follows:
As you can se boiling the water causes the precipitation of solid calcium carbonate or solid magnesium carbonate. This removes the calcium ions or magnesium ions from the water, and so removes the hardness. Therefore, hardness due to bi- carbonates is said to be temporary.
The permanent hardness i.e non-carbonate hardness is due to presence of sulphates, chlorides and nitrates of calcium and magnesium. It requires special methods of water softening.
Hardness is expressed in part per million as calcium carbonate or commonly known as ppm as calcium carbonate.
Water with hardness up to 50 ppm is known as soft water . 50 to 150 ppm it is termed as Medium and 150 to 300 ppm it is termed as moderately hard water . If the hardness is more than 300 ppm it is known as hard water.
Total hardness is commonly found by determining the amount of calcium and magnesium by a gravimetric analysis and by calculating their equivalent values in terms of CaCO
3
.
The most common testing method for hardness is the EDTA titrimetric method.
Disodium ethylenediamine tetra acetate (Na
2
EDTA) forms stable complex ions with
Ca
+2,
Mg
+2
, and other divalent cations causing hardness, and remove them from solution. When a small amount of Erichrome black T dye is added to the water containing hardness ions at pH 10, the solution becomes wine red and if there is no hardness the colour is blue. With the addition of EDTA the water sample having indicator dye starts forming stable complexes until all ions have been removed from solution and the water colour changes from wine red to blue indicating the end point.
Ca +2 + Mg +2 + EDAT PH=10 Ca.EDAT +Mg.EDATA
Wine red colour Blue Colour
Calcium hardness can be determined by increasing the pH value of water to 12, at which magnesium ions get precipitated and EDTA forms stable complex while reacting with calcium ions, resulting in change of colour from pink to purple when murexide is used as an indicator.
Apparatus
Burette.
Two Conical flasks
Pipette
Graduated cylinder
Funnel
Beaker
Hot plate stirrer
Reagents
(i)
Erichrome Black-T indicator.
Dissolve 0.2 gram of the dyestuff in 15 ml of Triethanolamine and 5 ml ethanol or dissolve 0.5 gm dyestuff in 100 ml of rectified spirit.
Its chemical formula can be written as HOC
10
H
6
N=NC
10
H
4
(OH)(NO
2
)SO
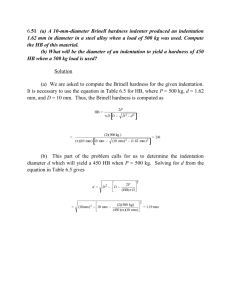
3
Na.
(ii) Ammonia buffer.
Dissolve 16.9 gram of Ammonium Chloride (NH
4
Cl) in 143 ml of concentrated ammonium hydroxide (NH
4
OH). Add 1.25 gram of magnesium salt of EDTA to obtain sharp change in colour of indicator and dilute to 250 ml with distilled water. One or two ml of this solution is required for raising the pH value of sample to 10.
(iii) Standard Ethylene diamine tetra acetic acid (E.D.T.A.)solution
0.01M.
Dissolve 3.723 gram EDTA sodium salt and dilute to 1000 ml.
(iv) Inhibitor.(
Adjust acid samples to pH 6 or higher with buffer or 0.1N
NaOH
)
Dissolve 4.5 gram of hydroxylamine hydrochloride in 100 ml of 95% ethyl alcohol or isopropyl alcohol.
Procedure:
(A) Total Hardness
1.
Pipette 25-ml of the tap water sample into a a conical flask (Erlenmeyer flask).
2.
Add at least one ml of Ammonia buffer solution. The pH should be 10. To check pH, standardize pH meter.
3.
Place the magnetic stirrer in the beaker and turn on the stirrer slowly.
4.
Add a few drops Eriochrome Black T indicator to the Erlenmeyer.
5.
Fill the burette with standardized EDTA. Record the initial burette reading.
6.
Immediately begin to titrate the sample two drops at a time. Be careful to titrate slowly near the endpoint, as the color will take about 5 seconds to develop. Thus, add the last few drops at 3-5 second intervals. The endpoint color is blue.
7.
Record the initial and final burette reading to the nearest 0.1 ml.
(B) Calcium Hardness
1.
Pipette 30-ml of the tap water sample into a a conical flask (Erlenmeyer flask).
2.
Add at least one ml of NaoH solution. The pH should be 12. To check pH, standardize pH meter.
3.
Place the magnetic stirrer in the beaker and turn on the stirrer slowly.
4.
Add a few drops a pinch of murexide indicator to the Erlenmeyer.
5.
Fill the burette with standardized EDTA. Record the initial burette reading.
6.
Immediately begin to titrate the sample two drops at a time. Be careful to titrate slowly near the endpoint, as the color will take about 5 seconds to develop. Thus, add the last few drops at 3-5 second intervals. The endpoint color is blue.
7.
Record the initial and final burette reading to the nearest 0.1 ml
Calculations
Total Hardness ( mg/L) as CaCO3 =
𝑉
𝐸𝐷𝑇𝐴
𝑉
× 1000 𝑠𝑎𝑚𝑝𝑙𝑒
Calcium (mg/L)as Ca +2 =
𝑉
𝐸𝐷𝑇𝐴
×400.8
𝑉 𝑠𝑎𝑚𝑝𝑙𝑒
Magnesium ( mg/L) as Mg +2 = Hardness( mg as CaCO3/L)/4.118 − 2.497 [ Ca +2 , mg/L]/4.118