Report - sages
advertisement

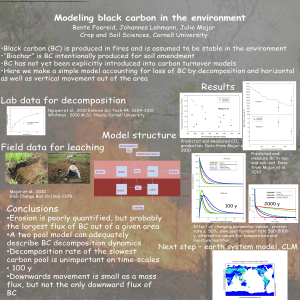
Report from exchange January 2013 Visit by Dr. Bente Foereid to CEBAS-CSIC, Murcia, Spain Photodegradation in nitrogen turnover in a semi-arid area Introduction Decomposition and nutrient release from plant residues depend on the chemical composition of the organic material as well as climatic and soil factors. The rate of nutrient release from decomposing residues in natural and agricultural systems can usually be predicted from those factors (Parton et al. 2007; Tian et al. 2007). However, in arid and semi-arid areas, decomposition is often much faster than predicted from known factors. Recently it has been discovered that exposure to light and UV light may play an important role in plant residue and soil organic matter decomposition and the carbon cycle in semi-arid areas (Pancotto et al. 2005; Austin and Vivanco 2006; Parton et al. 2007; Day et al. 2007; Vanderbilt et al. 2008; Foereid et al. 2011; Mayer et al. 2012). The mechanism for how photo-exposure affects residue mass loss remains obscure, but recent work suggests that it particularly affects lignin degradation, the plant compound most resistant to microbial degradation (Austin and Ballare 2010), and that photoexposure enhances subsequent microbial decomposition (Foereid et al. 2010) by making a larger part of the material available to early colonizers (Gallo et al. 2009). The effect of photo-exposure on nitrogen mineralization has been studied far less than the effect on carbon mineralization but there is some evidence that photo-exposure may substantially increase nitrogen release rate from plant residues (Parton et al. 2007; Foereid et al. 2010). In this collaboration with an institute in a semi-arid area we investigate the effect of photoexposure on carbon and nitrogen turnover during decomposition. We aim to try to understand it mechanistically and how plant litter of different initial quality responds. Work carried out Olive tree and maize samples had been left exposed to the sun together with shaded controls over the summer by the Spanish collaborator. During the visit, and incubation was performed where the olive tree, sun exposed and shaded samples were incubated both as whole pieces and grinded. Specialized equipment at the host institution was used to measure gas (carbon dioxide and nitrous oxide) evolution during the incubation, and soil mineral nitrogen content was measured. Soils for the incubation was sampled and prepared during the visit, and plant and soil samples were prepared for chemical analysis, also for a second experiment on maize residues to be performed later. Results and discussion The effect of photo-exposure on subsequent carbon mineralization and nitrous oxide emission (Figure) was smaller in this experiment than in my previous experiment (Foereid et al. 2010), and development of the incubation over time was erratic. There are several possible explanations for the difference from earlier results. The plant material used in the experiment was different (more recalsitrant in this experiment), the plant material was mixed into the soil and the plant material was exposed to the sun in plastic bags rather than to artificial light in the laboratory. The latter factor could be important, because also high temperature could affect the plant material, and the temperature in the plastic bags in the summer in Murcia would sometimes get very high. This would have affected both the sun-exposed and shaded treatments to some extent. Both the larger pieces of plant material as they were in the bags, and the same material grinded were incubated. This also impacted carbon and nitrogen fluxes (Figure). 40000 Carbon Dioxide -1 g CO2-C h kg soil 30000 -1 20000 10000 0 35 Nitrous Oxide 30 control, soil only sun-exposed, small pieces shaded, small pieces sun-exposed large pieces shade, large pieces 20 -1 -1 g N2O-N h kg soil 25 15 10 5 0 0 2 4 6 8 10 12 Days after adding water 14 16 18 Figure: Gas evolution during the incubation. Future prospects In addition to the incubation carried out during the visit, the groundwork was laid for future collaboration on photodegradation. Another incubation will be carried out using the maize samples, and isotope work is also planned. I also brought samples for X-ray computed tomography to determine any changes in three-dimensional microstructure caused by photoexposure. Planned output In addition to starting collaborations, one paper will follow directly from the work carried out during the visit: Cauyela & Foereid: Effect of photo-exposure on carbon and nitrogen turnover during decomposition of plant litter of different initial quality References Austin, A. T., & Ballare, C. L. (2010). Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proceedings of the National Academy of Sciences, 107, 46184622. Austin, A. T., & Vivanco, L. (2006). Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature, 442, 555-558. Day, T. A., Zhang, E. T., & Ruhland, C. T. (2007). Exposure to UV-B radiation accelerates mass and lignin loss of Larrea tridentata litter in the Sonoran Desert. Plant Ecology. Foereid, B., Bellarby, J., Meier-Augenstein, W., & Kemp, H. (2010). Does light exposure make plant litter more degradable? Plant and Soil, 333, 275-285. Foereid, B., Rivero, M. J., Primo, O., & Ortiz, I. (2011). Modelling photodegradation in the global carbon cycle. Soil Biology & Biochemistry, 43, 1383-1386. Gallo, M. E., Porras-alfaro, A., Odenbach, K. J., & Sinsabaugh, R. L. (2009). Photoaccelartation of plant litter decomposition in an arid environment. Soil Biology & Biochemistry, 41, 1433-1441. Mayer, L. M., Thornton, R. T., Schick, L. L., Jastrow, J. D., & Harden, J. W. (2012). Photodissolution of soil organic matter. Geoderma, 170, 314-321. Palm, C. A., Giller, K. E., Mafongoya, P. L., & Swift, M. J. (2001). Management of organic matter in the tropics:translating theory into practice. Nutrient Cycling in Agroecosystems, 61, 63-75. Pancotto, V. A., Sala, O. E., Robson, T. M., Caldwell, M. M., & Scopel, A. L. (2005). Direct and indirect effects of solar ultraviolet-B radiation on long-term decomposition. Global Change Biology, 11, 1982-1989. Parton, W., Silver, W. L., Burke, I. C., Grassens, L., Harmon, M. E., Currie, W. S., et al. (2007). Global-Scale Similarities in Nitrogen Release Patterns During Long-Term Decomposition. Science, 315, 361-364.