Table S1: Materials List
advertisement
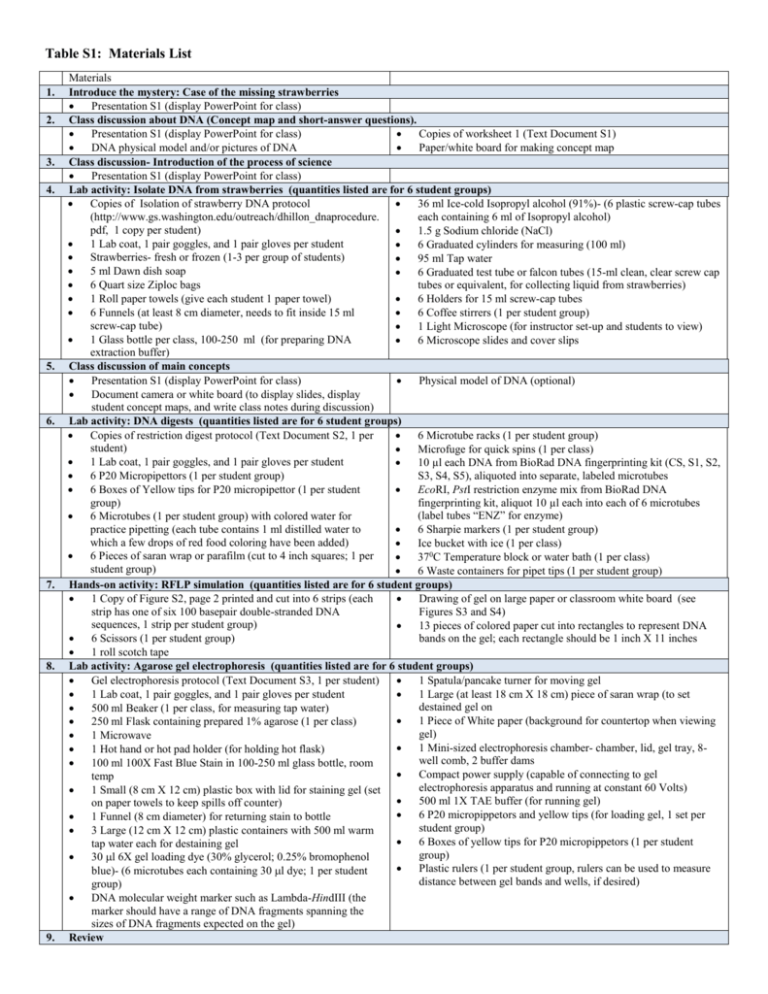
Table S1: Materials List 1. 2. 3. 4. 5. 6. 7. 8. 9. Materials Introduce the mystery: Case of the missing strawberries Presentation S1 (display PowerPoint for class) Class discussion about DNA (Concept map and short-answer questions). Presentation S1 (display PowerPoint for class) Copies of worksheet 1 (Text Document S1) DNA physical model and/or pictures of DNA Paper/white board for making concept map Class discussion- Introduction of the process of science Presentation S1 (display PowerPoint for class) Lab activity: Isolate DNA from strawberries (quantities listed are for 6 student groups) Copies of Isolation of strawberry DNA protocol 36 ml Ice-cold Isopropyl alcohol (91%)- (6 plastic screw-cap tubes (http://www.gs.washington.edu/outreach/dhillon_dnaprocedure. each containing 6 ml of Isopropyl alcohol) pdf, 1 copy per student) 1.5 g Sodium chloride (NaCl) 1 Lab coat, 1 pair goggles, and 1 pair gloves per student 6 Graduated cylinders for measuring (100 ml) Strawberries- fresh or frozen (1-3 per group of students) 95 ml Tap water 5 ml Dawn dish soap 6 Graduated test tube or falcon tubes (15-ml clean, clear screw cap 6 Quart size Ziploc bags tubes or equivalent, for collecting liquid from strawberries) 1 Roll paper towels (give each student 1 paper towel) 6 Holders for 15 ml screw-cap tubes 6 Funnels (at least 8 cm diameter, needs to fit inside 15 ml 6 Coffee stirrers (1 per student group) screw-cap tube) 1 Light Microscope (for instructor set-up and students to view) 1 Glass bottle per class, 100-250 ml (for preparing DNA 6 Microscope slides and cover slips extraction buffer) Class discussion of main concepts Presentation S1 (display PowerPoint for class) Physical model of DNA (optional) Document camera or white board (to display slides, display student concept maps, and write class notes during discussion) Lab activity: DNA digests (quantities listed are for 6 student groups) Copies of restriction digest protocol (Text Document S2, 1 per 6 Microtube racks (1 per student group) student) Microfuge for quick spins (1 per class) 1 Lab coat, 1 pair goggles, and 1 pair gloves per student 10 µl each DNA from BioRad DNA fingerprinting kit (CS, S1, S2, 6 P20 Micropipettors (1 per student group) S3, S4, S5), aliquoted into separate, labeled microtubes 6 Boxes of Yellow tips for P20 micropipettor (1 per student EcoRI, PstI restriction enzyme mix from BioRad DNA group) fingerprinting kit, aliquot 10 µl each into each of 6 microtubes (label tubes “ENZ” for enzyme) 6 Microtubes (1 per student group) with colored water for 6 Sharpie markers (1 per student group) practice pipetting (each tube contains 1 ml distilled water to which a few drops of red food coloring have been added) Ice bucket with ice (1 per class) 6 Pieces of saran wrap or parafilm (cut to 4 inch squares; 1 per 370C Temperature block or water bath (1 per class) student group) 6 Waste containers for pipet tips (1 per student group) Hands-on activity: RFLP simulation (quantities listed are for 6 student groups) 1 Copy of Figure S2, page 2 printed and cut into 6 strips (each Drawing of gel on large paper or classroom white board (see strip has one of six 100 basepair double-stranded DNA Figures S3 and S4) sequences, 1 strip per student group) 13 pieces of colored paper cut into rectangles to represent DNA 6 Scissors (1 per student group) bands on the gel; each rectangle should be 1 inch X 11 inches 1 roll scotch tape Lab activity: Agarose gel electrophoresis (quantities listed are for 6 student groups) Gel electrophoresis protocol (Text Document S3, 1 per student) 1 Spatula/pancake turner for moving gel 1 Lab coat, 1 pair goggles, and 1 pair gloves per student 1 Large (at least 18 cm X 18 cm) piece of saran wrap (to set destained gel on 500 ml Beaker (1 per class, for measuring tap water) 1 Piece of White paper (background for countertop when viewing 250 ml Flask containing prepared 1% agarose (1 per class) gel) 1 Microwave 1 Mini-sized electrophoresis chamber- chamber, lid, gel tray, 8 1 Hot hand or hot pad holder (for holding hot flask) well comb, 2 buffer dams 100 ml 100X Fast Blue Stain in 100-250 ml glass bottle, room Compact power supply (capable of connecting to gel temp electrophoresis apparatus and running at constant 60 Volts) 1 Small (8 cm X 12 cm) plastic box with lid for staining gel (set 500 ml 1X TAE buffer (for running gel) on paper towels to keep spills off counter) 6 P20 micropippetors and yellow tips (for loading gel, 1 set per 1 Funnel (8 cm diameter) for returning stain to bottle student group) 3 Large (12 cm X 12 cm) plastic containers with 500 ml warm 6 Boxes of yellow tips for P20 micropippetors (1 per student tap water each for destaining gel group) 30 l 6X gel loading dye (30% glycerol; 0.25% bromophenol Plastic rulers (1 per student group, rulers can be used to measure blue)- (6 microtubes each containing 30 l dye; 1 per student distance between gel bands and wells, if desired) group) DNA molecular weight marker such as Lambda-HindIII (the marker should have a range of DNA fragments spanning the sizes of DNA fragments expected on the gel) Review No materials needed 10. Student elaboration Computer(s) to view web resources in Table 2 If teaching the lesson without the lab, include steps 1,2,3,5,7,9,10. White board (for class discussion)







