Earth Energy Balance * Equilibrium and Conservation of Energy
advertisement

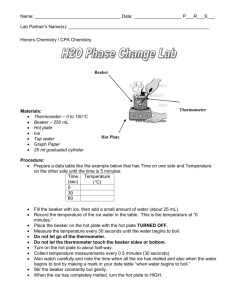
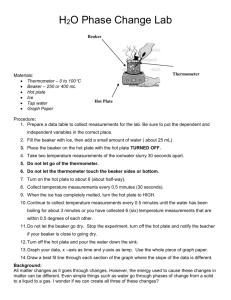
Earth Energy Balance Make-up Score:______/30 Name:_______________________________ Date:_________________Period:________ Beginning Question: Using a simple model of the Sun – Earth system to measure the transfer of thermal energy, does the system reach thermal equilibrium? Temperature Prediction: Sketch a graph predicting the change in temperature of the water in the beaker 0 15 Time (minutes) 30 Safety Considerations: Safety glasses must be worn by all group members for the entire lab. Use caution handling hot glassware and liquids. The hot plate stays very hot for a long time! Procedures: 1. Set up a data table to record time and temperature every minute for 30 minutes. 2. Add ~150 mL of water to the beaker. 3. Set up equipment as shown in the lab demo – do not turn on the hot plate. 4. Record the temperature of the water before the hot plate is turned on (time = 0 minutes). 5. Wait for the signal from your teacher, then turn on the hot plate ¼ to ½ turn only. 6. Record the temperature of the water at one minute intervals for 15 minutes. 7. Place a watch glass on the beaker. Continue recording temperatures for 15 more minutes. 8. Turn off the hot plate and clean up your materials. Data, Observations, Calculations and Graphs: 1. What does the hot plate represent in your system? What does the beaker of water represent? 2. What does adding the watch glass to your system represent? Time (min) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Temperature (°C) 22 23 24 25 27 29 31 34 37 40 44 45 45 45 45 45 Time (min) 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Temperature (°C) 46 47 49 51 52 53 54 55 56 56 56 56 57 57 57 3. Construct a graph of the time and temperature data. 4. Describe the trend in your data. Be sure to talk about any plateaus (times when the temperature stayed the same) in your curve, and what happens to the temperature of the system when the watch glass is added. Claims and Evidence: Answer in Complete Sentences! 5. Did the system reach thermal equilibrium? Use your graph to explain how you know the system was in equilibrium. 6. Compare the amount of energy flowing into the system with the amount of energy flowing out of the system when the system reaches thermal equilibrium. Errors and Improvements: Reflection: 1. Does your data support your prediction? Explain by comparing your graph from your prediction with the graph of your actual lab data. 2. What do the hot plate, beaker of water and watch glass model in the Sun – Earth system?