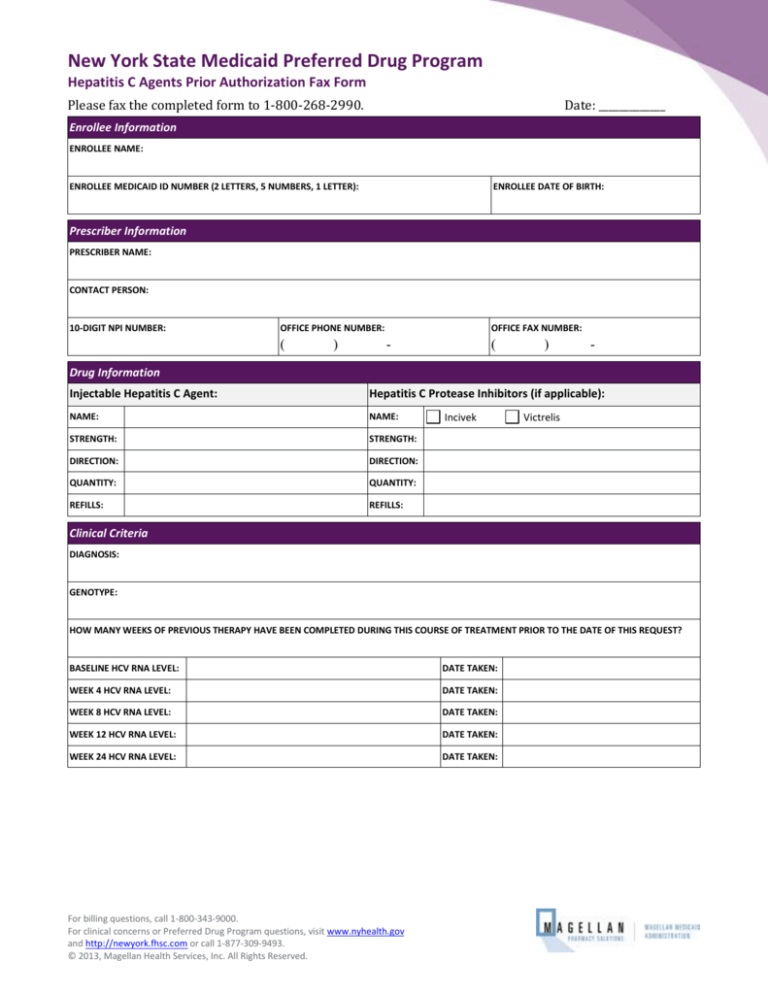
New York State Medicaid Preferred Drug Program
Hepatitis C Agents Prior Authorization Fax Form
Please fax the completed form to 1-800-268-2990.
Date: _____________
Enrollee Information
ENROLLEE NAME:
ENROLLEE MEDICAID ID NUMBER (2 LETTERS, 5 NUMBERS, 1 LETTER):
ENROLLEE DATE OF BIRTH:
Prescriber Information
PRESCRIBER NAME:
CONTACT PERSON:
10-DIGIT NPI NUMBER:
OFFICE PHONE NUMBER:
(
)
OFFICE FAX NUMBER:
-
(
)
-
Drug Information
Injectable Hepatitis C Agent:
Hepatitis C Protease Inhibitors (if applicable):
NAME:
NAME:
STRENGTH:
STRENGTH:
DIRECTION:
DIRECTION:
QUANTITY:
QUANTITY:
REFILLS:
REFILLS:
Incivek
Victrelis
Clinical Criteria
DIAGNOSIS:
GENOTYPE:
HOW MANY WEEKS OF PREVIOUS THERAPY HAVE BEEN COMPLETED DURING THIS COURSE OF TREATMENT PRIOR TO THE DATE OF THIS REQUEST?
BASELINE HCV RNA LEVEL:
DATE TAKEN:
WEEK 4 HCV RNA LEVEL:
DATE TAKEN:
WEEK 8 HCV RNA LEVEL:
DATE TAKEN:
WEEK 12 HCV RNA LEVEL:
DATE TAKEN:
WEEK 24 HCV RNA LEVEL:
DATE TAKEN:
For billing questions, call 1-800-343-9000.
For clinical concerns or Preferred Drug Program questions, visit www.nyhealth.gov
and http://newyork.fhsc.com or call 1-877-309-9493.
© 2013, Magellan Health Services, Inc. All Rights Reserved.
Magellan Medicaid Administration
Hepatitis C Agents Prior Authorization Fax Form
YOU WILL NEED TO COMPLETE ONLY ONE OF THE FOLLOWING THREE BOXES, and then sign
the attestation that follows
Please answer all of the following for
Triple Therapy with Incivek, peginterferon, and ribavirin:
Has the patient previously failed therapy with Incivek or Victrelis?
Yes
No
Will the patient be on peginterferon and ribavirin in combination with Incivek?
Yes
No
Is HCV RNA ≤1000 IU/mL at week 4?
Yes
No
Is HCV RNA ≤1000 IU/mL at week 12?
Yes
No
Is HCV RNA undetectable at both week 4 and week 12?
Yes
No
Is HCV RNA detectable but ≤1000 IU/mL at either week 4 or week 12?
Yes
No
Please check the box that best describes the patient:
Treatment-naïve without cirrhosis
Prior relapser (achieved undetectable HCV RNA at end of previous treatment with peginterferon and ribavirin but detectable within
24 weeks after treatment)
Prior partial responder (≥2 log decrease in HCV RNA at week 12 of previous treatment with peginterferon and ribavirin but did not
achieve undetectable HCV RNA at end of treatment)
Prior null responder (achieved <2 log decrease in HCV RNA at week 12 of previous treatment with peginterferon and ribavirin)
Treatment-naïve with compensated cirrhosis
Please answer all of the following for
Triple therapy with Victrelis, peginterferon, and ribavirin:
Has the patient previously failed therapy with Incivek or Victrelis?
Yes
No
Will the patient be on peginterferon and ribavirin in combination with Victrelis?
Yes
No
Did the patient complete four consecutive weeks of therapy with ribavirin and peginterferon within 30 days of
the initial request?
Yes
No
Is HCV RNA undetectable at week 8 (= week 8 of peginterferon and week 4 of Victrelis)?
Yes
No
Is HCV RNA <100 IU/mL at week 12 (= week 12 of peginterferon and week 8 of Victrelis)?
Yes
No
Is HCV RNA undetectable at week 24 (= week 24 of peginterferon and week 20 of Victrelis)?
Yes
No
Please check the box that best describes the patient:
Treatment-naïve without cirrhosis
Prior relapser (achieved undetectable HCV RNA at end of previous treatment with peginterferon and ribavirin but detectable within
24 weeks after treatment)
Prior partial responder (≥2 log decrease in HCV RNA at week 12 of previous treatment with peginterferon and ribavirin but did not
achieve undetectable HCV RNA at end of treatment)
Prior null responder (achieved <2 log decrease in HCV RNA at week 12 of previous treatment with peginterferon and ribavirin)
Treatment-naïve with compensated cirrhosis
Revision Date: February 6, 2016
For billing questions, call 1-800-343-9000.
For clinical concerns or Preferred Drug Program questions, visit
www.nyhealth.gov and http://newyork.fhsc.com or call 1-877-309-9493.
Page 2
Magellan Medicaid Administration
Hepatitis C Agents Prior Authorization Fax Form
Please answer all of the following for Injectable Hepatitis C agents when used with ribavirin only
DUAL
THERAPY:
Will the patient be on ribavirin in combination with the Injectable Hepatitis C Agent?
Yes
No
Please check the box that demonstrates the patient’s response at week 12:
No early virologic response (EVR) [HCV RNA decreased < 2 log]
Partial EVR [HCV RNA decreased ≥2 log]
Complete EVR [HCV RNA negative]
Please check the box that demonstrates the patient’s response at week 24:
HCV RNA negative
HCV RNA positive
If requesting Injectable Hepatitis C treatment for genotype 2 or 3 beyond 24 weeks, please answer the following:
Does the patient have a comorbidity requiring adjustment to the expected duration of therapy for patients with genotype 2 and 3?
Yes
No
If yes, list comorbid condition(s):
If requesting Injectable Hepatitis C treatment beyond 48 weeks, please answer the following:
Has the patient demonstrated a delayed virologic response (partial EVR at week 12 and HCV RNA negative at week 24)?
Yes
No
I attest that this is medically necessary for this patient and that all of the information on this form is
accurate to the best of my knowledge. I attest that documentation of the above diagnosis and medical
necessity is available for review if requested by New York Medicaid.
PRESCRIBER’S SIGNATURE
Revision Date: February 6, 2016
DATE
For billing questions, call 1-800-343-9000.
For clinical concerns or Preferred Drug Program questions, visit
www.nyhealth.gov and http://newyork.fhsc.com or call 1-877-309-9493.
Page 3
Boceprevir (Victrelis®)
Boceprevir is a new drug for the treatment of chronic hepatitis C virus (HCV) genotype 1. It is a member of the HCV
protease inhibitor class and represents the first direct-acting antiviral therapy for hepatitis C. It is meant to be an adjunct
to the mainstay of HCV therapy and used concurrently with both peginterferon and ribavirin (PR). 1
Advantages of adding boceprevir to therapy
Boceprevir was designed to inhibit HCV NS3/4A which prevents the cleavage of viral polyproteins during HCV replication.
It is a complementary therapy to PR that together enhances host defenses against the virus. It has been shown to increase
sustained virologic response (SVR) rates in both treatment-naïve and treatment-experienced patients when compared to
using standard treatment with only PR.
Trial
Subjects
Treatment arm
including lead-in*
Overall SVR rate (%)
SVR rate (%)
Non-black patients
SVR rate (%)
Black patients
SPRINT-22
1,097
treatmentnaïve
B24 + PR28 or 48
63 (p<0.001)
67 (p<0.001)
53 (p<0.001)
B44 + PR48
66 (p<0.001)
68 (p<0.001)
42 (p=0.004)
PR48 (Control)
38
40
23
403
treatmentexperienced
Treatment arm
including lead-in*
Overall SVR rate (%)
SVR rate (%)
Prior relapsers
SVR rate (%)
Prior partial responder
B32 + PR36 or 48
59 (p<0.001)
69 (p<0.001)
40 (p<0.001)
B44 + PR48
66 (p<0.001)
75 (p<0.001)
52 (p<0.001)
PR48 (Control)
21
29
7
RESPOND-23
*by weeks on each component; B=boceprevir; PR=peginterferon + ribavirin; lead-in=4 wks of PR prior to adding B or placebo;
p values are in reference to the statistical significance as compared to the control group
Cautions
When boceprevir is used as monotherapy the development of treatment-emergent resistance mutations occurs
rapidly. Resistance is also seen in patients that do not achieve SVR with combination therapy.
Boceprevir should not be used if a patient has previously failed treatment with another protease inhibitor (such as
telaprevir) as there is cross-resistance.
Most common adverse reactions with boceprevir are fatigue, anemia, nausea, headache, and dysgeusia.
Worsening anemia and neutropenia can occur when boceprevir is added to PR and may require treatment or
discontinuation of the drug.
Clinically significant drug interactions must be considered; boceprevir is partially metabolized via CYP3A4/5 and pglycoprotein; co-administration with ritonavir-boosted HIV protease inhibitors is not recommended.
Where does boceprevir fit into therapy?
The American Association for the Study of Liver Diseases recently updated guidelines for treating genotype 1 chronic HCV
to recommend either boceprevir or telaprevir in combination with PR as optimal therapy in treatment-naïve and
treatment-experienced patients.4 Many patient-specific factors must be taken into consideration when deciding to initiate
therapy. The goal in treating HCV is to cure the infection as evidenced by SVR, an undetectable HCV RNA level 24 weeks
after treatment has ended. SVR is generally associated with resolution of liver disease in patients without cirrhosis as well
as improvements in morbidity and mortality.
How it should be used
Baseline genotype must be established by testing as boceprevir is only approved in HCV genotype 1. Effective use of
boceprevir is dependent on response-guided therapy. Duration of treatment is determined by response and previous
treatment status. It is essential to assess response by testing HCV-RNA viral load at critical points: after a 4 week lead in
period of PR (week 4 of treatment), and then at treatment weeks 8, 12, and 24.
Boceprevir (Victrelis®) product labeling. Schering Corporation, a subsidiary of Merck & Co., Inc. Whitehouse Station, NJ; May 2011.
Poordad F, et al.; for SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364: 1195-1206.
3 Bacon BR, et al.; for HCV RESPOND-,2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011; 364: 1207-1217.
4 Ghany M, et al. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guidelines by the American Association for the Study of Liver
Diseases. Hepatology. Sep 2, 2011.
2
http://nypep.nysdoh.suny.edu • E-mail: PEP@nysdoh.suny.edu
Boceprevir initiation and monitoring
Once patient readiness for chronic hepatitis C treatment has been determined, the algorithm below outlines key decision
points for initiating and monitoring combination therapy including boceprevir. This algorithm is available in interactive
format on the NYMPEP website at: http://nypep.nysdoh.suny.edu.
Note: Ribavirin is contraindicated in pregnancy therefore all female patients of childbearing age (or female partners of
male patients) should be sure they are not pregnant prior to beginning treatment and should use two methods of nonhormonal birth control throughout treatment.
Has the patient been diagnosed with HCV genotype 1
and received quantitative HCV RNA testing?
No
Seek alternative treatment
options or conduct testing
prior to treatment.
Yes
Initiate a 4-week lead-in treatment period with
peginterferon alpha and ribavirin. Has the patient
completed 4 consecutive weeks of lead-in therapy?
No
Provide 4 consecutive weeks of
lead-in therapy prior to
initiating boceprevir.
Yes
At the end of treatment week 4, add boceprevir 800
mg three times daily to peginterferon alpha and
ribavirin and obtain quantitative HCV RNA. Repeat
quantitative HCV RNA at treatment weeks 8 and 12.
Is HCV RNA <100 IU/mL at week 12?
No
Stop treatment in all patients.
No further HCV RNA testing.
Yes
No
Repeat HCV RNA at week 24. Is HCV RNA
undetectable at week 24?
Yes
Is the patient
Is the patient
O
O
treatment-naïve
prior partial
R
without cirrhosis?
responder?* R
Is the patient
prior
relapser?+
No
Prior null O Treatment-naïve
responder?‡ R w/ compensated
cirrhosis?
Yes
Was HCV RNA undetectable at treatment week 8?
No
Yes
Continue triple therapy to the end
of week 28 in treatment naïve
patients. Continue triple therapy to
the end of week 36 in prior partial
responders or prior relapsers.
Yes
Continue triple therapy to the
end of week 36. Continue
peginterferon alpha & ribavirin
to the end of week 48.
Continue triple therapy to the
end of week 48.
Obtain HCV RNA 24 weeks after the end of treatment to
determine sustained virological response.
*Prior partial responder = achieved ≥2 log decrease in HCV RNA at week 12 of previous treatment with peginterferon and ribavirin but did not achieve
undetectable HCV RNA at end of treatment
+Prior relapser = achieved undetectable HCV RNA at end of previous treatment with peginterferon and ribavirin but detectable within 24 weeks after
treatment
‡Prior null responder = achieved <2 log decrease in HCV RNA at week 12 of previous treatment with peginterferon and ribavirin
http://nypep.nysdoh.suny.edu • E-mail: PEP@nysdoh.suny.edu
Telaprevir (Incivek®)
Telaprevir is a new drug for the treatment of chronic hepatitis C virus (HCV) genotype 1. It is a member of the HCV
protease inhibitor class and represents the first direct-acting antiviral therapy for hepatitis C. It is meant to be an
adjunct to the mainstay of HCV therapy and used concurrently with both peginterferon and ribavirin (PR) in adults. 1
Advantages of adding telaprevir to therapy
Telaprevir was designed to inhibit HCV NS3/4A which prevents the cleavage of viral polyproteins during HCV
replication. It is a complementary therapy to PR that together enhances host defenses against the virus. It has been
shown to increase the sustained virologic response (SVR) in both treatment-naïve and treatment-experienced
patients when compared to using only PR.
Trial
Subjects
Treatment arm*
ADVANCE2
1,088
treatment naïve
ILLUMINATE3
540
treatment naïve
T12+PR24/48
T8+PR24/48
PR48 (control)
T12+PR12 to start (all)
e-RVR T12+PR24
e-RVR T12+PR48
non-e-RVR T12+PR48
REALIZE4
662
treatment experienced
Treatment arm*
T12+PR48
Lead-in T12+PR48
PR48 (control)
Overall SVR rate (%)
Overall
rate
64 (p<0.001)
66 (p<0.001)
17
75 (p<0.001)
69 (p<0.001)
44
72
92 (non-inferior to e-RVR T12+PR48)
88
64
Prior
Prior partial
Prior null
relapsers
responders
responders
83 (p<0.001)
59 (p<0.001)
29 (p<0.001)
88 (p<0.001)
54 (p<0.001)
33 (p<0.001)
24
14
5
*by weeks on each component; T=telaprevir; PR=peginterferon + ribavirin; e-RVR=extended rapid virologic response (undetectable HCV RNA at wks 4 & 12);
Lead-in=4 wks PR prior to adding T; p values are in reference to the statistical significance as compared to the control group
Cautions
When telaprevir is used as monotherapy the development of treatment-emergent resistance mutations occurs
rapidly. Resistance is also seen in patients that do not achieve SVR with combination therapy.
Telaprevir should not be used if a patient has previously failed treatment with another HCV NS3/4A protease
inhibitor (such as boceprevir) as there is cross resistance.
Higher rates of anemia, rash and pruritus occurred in telaprevir treated patients and rash was the most common
reason for treatment discontinuation.
Clinically significant drug interactions must be considered; telaprevir is a substrate and inhibitor of CYP3A and pglycoprotein; co-administration with some HIV protease inhibitors is not recommended.
Where does telaprevir fit into therapy?
The American Association for the Study of Liver Diseases recently updated guidelines for treating genotype 1 chronic
HCV to recommend either boceprevir or telaprevir in combination with PR as optimal therapy in treatment-naïve
and treatment-experienced patients.5 Many patient-specific factors must be taken into consideration when deciding
to initiate therapy. The goal of treatment is to cure the infection as evidenced by SVR, an undetectable HCV RNA
level 24 weeks after treatment has ended. SVR is generally associated with resolution of liver disease in patients
without cirrhosis as well as improvements in morbidity and mortality.
How it should be used
Baseline genotype must be established as telaprevir is only approved in HCV genotype 1. Effective use of telaprevir is
dependent on response-guided therapy. Telaprevir should be given with PR for the first 12 weeks and PR continued
for 12-36 weeks after telaprevir therapy has ended, depending on response and previous treatment status. It is
essential to assess HCV RNA viral load at weeks 4 and 12 to determine duration of treatment.
Telaprevir (Incivek) product labeling. Vertex Pharmaceuticals, Inc. Cambridge, MA; May 2011.
Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. Jun 23 2011;364(25):2405-2416.
Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. Sep 15 2011;365(11):1014-1024.
4
Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. Jun 23 2011;364(25):2417-2428
5
Ghany M, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guidelines by the American Association for the Study of Liver Diseases. Hepatology. Sep 2,
2011.
2
3
http://nypep.nysdoh.suny.edu • E-mail: PEP@nysdoh.suny.edu
Telaprevir initiation and monitoring
Once patient readiness for chronic hepatitis C treatment has been determined, the algorithm below outlines key
decision points for initiating and monitoring combination therapy including telaprevir. This algorithm is available in
interactive format on the NYMPEP website at: http://nypep.nysdoh.suny.edu.
Note: Ribavirin is contraindicated in pregnancy therefore all female patients of childbearing age (or female partners of
male patients) should be sure they are not pregnant prior to beginning treatment and should use two methods of nonhormonal birth control throughout treatment.
*Prior partial responder = achieved ≥2 log decrease in HCV RNA at week 12 of previous treatment with peginterferon and ribavirin
but did not achieve undetectable HCV RNA at end of treatment
+Prior relapser = achieved undetectable HCV RNA at end of previous treatment with peginterferon and ribavirin but detectable
within 24 weeks after treatment
‡Prior null responder = achieved <2 log decrease in HCV RNA at week 12 of previous treatment with peginterferon and ribavirin
http://nypep.nysdoh.suny.edu • E-mail: PEP@nysdoh.suny.edu









