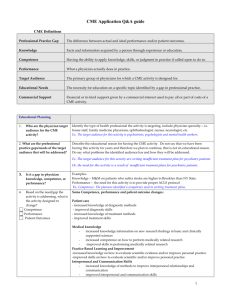
a more detailed programme

Navigating Compliance Waters:
CME/CPD Accreditation Criteria and Industry Codes
Tuesday 11 th December 2012
London, United Kingdom
The Queen Elizabeth II Conference Centre http://qeiicc.co.uk/compliance
Program
09.00
– 09.30 Registration
09:30
– 09:40 Welcome and Introductions: It’s a Changing World
After participating in this educational activity, participants should be able to:
Assess the guiding principles in the changing world of continuing medical education (CME) and continuing professional development (CPD)
Be familiar with CME/CPD accreditation criteria and pharmaceutical/device industry codes
Debate the impact on medical events and education
Discuss the opportunities and benefits that the changes create for your association
09.40 – 09.55 Guiding Principles
Julie Simper, CME/CPD Manager, Kenes Education
Quality and Effective Education
The value of traditional CME is being questioned
…Is it too focused on credits and certificates? Is knowledge actually being translated into improved clinical performance and improved patient outcomes? From CME to CPD, to revalidation and maintenance of certification.
Independence
What is the role of industry in accredited medical education? Is content commercially biased? Is medical education too often “marketing in the guise of education”?
Transparency
It’s not only important to do the right thing, but to show that you’re doing it and proactively communicate information.
09.55
– 10.15 CME/CPD Accreditation: Beyond Credits to Continuing Professional Development
Eugene Pozniak, European CME Forum, Good CME Practice Group
Doctors are under increasing pressure to engage in CME-accredited education, whether through national regulatory or re-licensing expectations or through their membership societies and associations. Accreditation standards in European
CME are evolving rapidly to accommodate these developments which are representative of a global trend. But each system has its own requirements and expectations. Eugene Pozniak will review some of the major differences and overall themes from the major accreditation bodies, including the new 2013 accreditation standards from the UEMS-
EACCME.
10.15 – 10.30 Education From the Specialty Society Perspective
Verity Baker, Project Manager, UK Stroke Forum
A case study of the UK Stroke Forum conference will be presented discussing the accreditations historically sought and awarded and how and why these have changed over time. Additionally, Ms. Baker will explain the UK Stroke
Forum Education and Training, as it relates to the changing emphasis within learning, beyond traditional meetings and conferences. We’ll explore how these types of disease-specific accreditations can sit alongside traditional CPD/CME systems, how to assess the importance of different accreditations to delegates, and at the same time prepare them and the conference for changes in learning and development.
10.30
– 10.45 Question and Answer Session with Panel
10.45 – 11.15 Refreshment Break and Optional Tour of Queen Elizabeth II Conference Centre
11.15
– 11.45 Industry Codes and European Event Pre-Assessment Systems
Julie Simper, CME/CPD Manager, Kenes Education
Nicky Simpson, Associate Director Congress, Astellas Pharma Europe
Christine Sainvil, EthicalMedTech Compliance Officer
Often tarred with negative perceptions -- warranted or not - of lavish golf weekend junkets and improper influencing of health care professionals (HCPs) by industry companies, the emphasis at medical meetings should, understandably so, be on the science, education, and professional networking. In an effort to ensure that the focus is indeed on content, changes are taking place in Europe that are forcing organisations to reassess how medical education meetings are conceptualised and delivered.
EFPIA e4ethics from the PCO viewpoint
Eucomed EthicalMedTech
Navigating Compliance Waters:
CME/CPD Accreditation Criteria and Industry Codes
Tuesday 11 th December 2012
London, United Kingdom
The Queen Elizabeth II Conference Centre http://qeiicc.co.uk/compliance
11.45 – 12.00 Question and Answer Session with Panel
12:00
– 12.45 Interactive Panel Discussion:
How have the changes in medical events and education affected your daily business?
What are key challenges and/or opportunities?
What do you think the future holds?
Specialty Associations
Verity Baker, Project Manager, UK Stroke Forum
Nicola Heard, Head of Education and Membership Services, Assoc. of Anaesthetists of Great Britain and Ireland
Pharmaceutical Industry
Jane Landles, Secretary to the Prescription Medicines Code of Practice Authority
Nicky Simpson, Associate Director Congress, Astellas Pharma Europe
Device/Tech Industry
Christine Sainvil, EthicalMedTech Compliance Officer
CME/CPD Providers
Eugene Pozniak, European CME Forum, Good CME Practice Group
Julie Simper, CME/CPD Manager, Kenes Education (moderator)
12:45
– 13:00 Summary and Conclusions
13.00 – 14.00 Lunch and Networking
Faculty
Verity Baker
Project Manager
UK Stroke Forum
Worcestershire, United Kingdom
Verity.Baker@stroke.org.uk
Nicola Heard
Head of Education and Membership Services
Association of Anaesthetists of Great Britain and Ireland
(AAGBI)
The AAGBI Foundation THE AAGBI FOUNDATION
London, United Kingdom nicolaheard@aagbi.org
Jane Landles
Secretary of the Prescription Medicines Code of Practice
Authority
London, United Kingdom
JLandles@pmcpa.org.uk
Eugene Pozniak
Managing Director, Siyemi Learning
Programme Director, European CME Forum
Founding Member, Good CME Practice Group epozniak@siyemi.org
Christine Sainvil
EthicalMedTech Compliance Officer
Brussels, Belgium
Christine.Sainvil@ethicalmedtech.eu
Nicky Simpson
Associate Director Congress
Astellas Pharma Europe
Julie Simper
CME/CPD Manager
Kenes Education
Amsterdam, the Netherlands jsimper@kenes.com
Navigating Compliance Waters:
CME/CPD Accreditation Criteria and Industry Codes
Tuesday 11 th December 2012
London, United Kingdom
The Queen Elizabeth II Conference Centre http://qeiicc.co.uk/compliance
Related Resources
Good CME Practice Group (gCMEg) www.gcmep.eu
The aim of the gCMEp Group is to look specifically at how the European education provider/agency community works in CME and to develop the appropriate operating standards. The results of the work of the gCMEp group have been published in a recent article: Setting CME standards in Europe: Guiding Principles for Medical Education, October 2012. The group is now opening up its membership to European education providers
European CME Forum (ECMEF) www.europeancmeforum.eu
The European CME Forum is dedicated to bringing together all stakeholder groups with an interest in European Continuing Medical
Education, promoting multi-channel discussion in an independent and neutral environment. The 6th annual meeting of the
European CME Forum will be held in London, 14-15 November 2013. You can find presentations from the 2012 annual meeting on the ECMEF website.
Alliance for Continuing Education in the Health Professions (ACEHP) www.acehp.org
The Alliance for Continuing Education in the Health Professions is a community of professionals dedicated to accelerating excellence in health care performance through education, advocacy, and collaboration. Founded in 1975 in support of certified medical education for physicians, the Alliance expanded its scope in 2010 to include health care-related continuing education and continuing professional development. Through inclusive, collaborative, and inter-professional efforts, the Alliance acts to close health care performance gaps and improve quality in patient care and in clinical outcomes.
General Medical Council (GMC) www.gmc-uk.org
The GMC has statutory role to promote high standards and co-ordinate all aspects of medical education. This includes doctors' continuing professional development. Good Medical Practice requires doctors to keep their knowledge and skills up to date throughout their working life and to maintain and improve their performance. Continuing professional development (CPD) is a key way for doctors to meet these professional standards and is one of the sources of information required for appraisal and revalidation. The GMC has published a guidance booklet for doctors - Continuing Professional Development: guidance for all doctors . The GMC does not endorse or accredit CPD.
For information on revalidation, see the Revalidation section.
Royal College of Physicians www.rcplondon.ac.uk
We support our fellows and members during every stage of their career and thus improve the quality of patient care. By setting and monitoring the standards of medical training, we ensure that patients are seen by fully trained, capable doctors. Our evidencebased guidelines and audits support our fellows and members in improving and scrutinising clinical care. Our education programmes provide physicians with the knowledge and skills they need for high performance.
European Accreditation Council for Continuing Medical Education (UEMS-EACCME) www.uems.net www.eaccme.net
The European Union of Medical Specialists (UEMS) was founded in 1958 with the aim of representing the interests of specialist doctors at an international level. The UEMS established the European Accreditation Council for Continuing Medical Education
(EACCME®), in January 2000, with the aim of encouraging high standards in the development, delivery and harmonisation of continuing medical education (CME). This was to be achieved through the international accreditation of CME events, and the establishment of a system for the international acceptance of CME credit points.
EthicalMedTech Conference Vetting System www.ethicalmedtech.eu
EthicalMedTech is a platform dedicated to ethics and compliance projects in the European MedTech industry and is supported by
Eucomed, the European medical technology industry association. EthicalMedTech currently focuses on the Conference Vetting
System established in 2012. This independent system reviews the compliance of third-party educational conferences with the
Eucomed Code of Ethical Business Practice.
Navigating Compliance Waters:
CME/CPD Accreditation Criteria and Industry Codes
Tuesday 11 th December 2012
London, United Kingdom
The Queen Elizabeth II Conference Centre http://qeiicc.co.uk/compliance
Eucomed www.eucomed.com
Eucomed represents the medical technology industry in Europe. Their mission is to make modern, innovative and reliable medical technology available to more people. Eucomed members include both national and pan-European trade and product associations as well as medical technology manufacturers. In total Eucomed represents around 22,500 designers, manufacturers and suppliers of medical technology used in the diagnosis, prevention, treatment and amelioration of disease and disability. e4ethics: Educational Events and Ethical Evaluation www.efpia-e4ethics.eu
The e4ethics system was introduced in 2011 as a service to EFPIA members to facilitate compliance reviews and pre-assessment of events with regard to the EFPIA Healthcare Professionals (HCP) Code. Reports are posted on the e4ethics online database and proactively communicated to event organisers, EFPIA members, and other relevant industry groups.
European Federation of Pharmaceutical Industries and Alliances (EFPIA) www.efpia.eu
The European Federation of Pharmaceutical Industries and Associations (EFPIA) represents the pharmaceutical industry operating in Europe. Through its direct membership of 33 national associations and 37 leading pharmaceutical companies, EFPIA is the voice on the EU scene of 1,900 companies committed to researching, developing and bringing to patients new medicines that will improve health and the quality of life around the world.
Prescription Medicines Code of Practice Authority (PMCPA) www.pmcpa.org.uk
The PMCPA is a selfregulatory body which administers the Association of the British Pharmaceutical Industry’s (ABPI) Code of
Practice for the Pharmaceutical Industry at arm’s length of the ABPI. Offers advice, guidance, and training (including an interactive elearning module for health professionals), along with a complaints procedure.