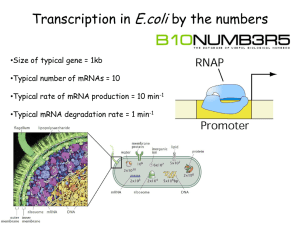
报告清单:
advertisement

学术报告: 费聪(李林组)Lys29-Linked Nonproteolytic Poly-Ubiquitination of Axin by Smurf1 Negatively Regulates Wnt/β-Catenin Signaling 苟兰涛(刘默芳组)Mouse piRNAs, in complex with MIWI and CAF1, mediate mRNA clearance in late stage of spermatogenesis 胡西旵(李伯良组)Suppression of HCC by inhibiting a specific cholesterol metabolic pathway to increase cytotoxic oxysterols 黄骞(王恩多组)Identification of essential nucleotides in tRNALeu to its functions by using a constructed yeast tRNALeu knockout strain 柳童斐(宋保亮组)Ablation of gp78 in liver improves hyperlipidemia and insulin resistance by inhibiting SREBP to decrease lipid biosynthesis 牟世荣(惠静毅组)Regulation of pre-mRNA alternative splicing by a multifunctional protein YB-1 谢盛松(张永莲组)Caput epididymis-specific Cre transgenic mouse model and functions of miR-34a in spermatogenesis 殷庆飞(陈玲玲组)Long noncoding RNAs with snoRNA ends 张润瑞(徐国良组)A Positive Role of Tet1 DNA Dioxygenase in the Regulation of AdultNeural Progenitor Cell Proliferation 赵雅(吴立刚组)microRNA serves as a mRNA surveillance system by eliminating nonsense messages 朱长兰(程红组)Premature Termination Codons Are Recognized in the Nucleus in A Reading-Frame Dependent Manner Lys29-Linked Nonproteolytic Poly-Ubiquitination of Axin by Smurf1 Negatively Regulates Wnt/β-Catenin Signaling Abstract Ubiquitination plays an important role in modulating protein functions. As a C2-WW-HECT type ubiquitin ligase, Smurf1 regulates a number of signaling pathways by promoting ubiquitin-dependent degradation of its targeted proteins. Here, we disclosed a nonproteolytic role of Smurf1 in regulating the function of Axin protein in Wnt/β-catenin signaling. Our data demonstrate that Smurf1 interacts with Axin in a noncanonical pattern, where its WW domains are not required, and ubiquitinates Axin via Lys29 (K29)-linked poly-ubiquitin chains. Smurf1-mediated Axin poly-ubiquitination disrupts Axin-LRP6 interaction, attenuates Wnt-stimulated LRP6 phosphorylation and inhibits Wnt/β-catenin signaling. Furthermore, Smurf1-Axin interaction and Axin ubiquitination are attenuated in the G2/M phase of cell cycle, resulting in an increase in the response to Wnt stimulation at that stage. Thus, our study unraveled a previously unappreciated role of Smurf1 in regulating protein functions in a cell cycle-dependent manner. Mouse piRNAs, in complex with MIWI and CAF1, mediate mRNA clearance in late stage of spermatogenesis Lan-Tao Gou, Yun-Ping Hu, Peng Dai, Li-Gang Wu, En-Duo Wang, and Mo-Fang Liu State Key Laboratory of Molecular Biology–Graduate School of Chinese Academy of Sciences, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 200031, China A unique feature in late stages of male-germ cell development is the remarkable nuclear morphological change and cytoplasm elimination. In mature sperm, little mRNAs retain; however, how the diverse and massive unwanted mRNAs in late stage of spermatids are eliminated during sperm maturation remains largely unexplored. Here, we show that piRNAs, to form pi-ribonucleoproteins (piRNPs) complexes with murine PIWI (MIWI) and deadenylase CAF1, induce mRNA degradation through deadenylation by imperfectly base-pairing with the 3-untranslated region (UTR) of mRNAs in mouse testes. Interestingly, we found that the deadenylation and degradation of piRNA target mRNAs occurred in elongating spermatids, suggesting that piRNPs complexes mediate mRNA clearance in late stage of spermatid development. In support of this, inhibition of piRNPs complexes led to up-regulation of all validated piRNA targets as well as stabilization of hundreds of predicted piRNA target mRNAs in elongating spermatids. Thus, our results support a model in which piRNAs utilize their enormous repertoire of targeting capacity to induce the degradation of massive mRNA transcripts in elongating spermatids, providing a novel mechanism for mRNA clearance in late stages of male-germ cell development. Moreover, our findings also indicate novel function of piRNAs in regulating protein genes in addition to acting as the vanguard of genome defence in mammalian germ cells. Suppression of HCC by inhibiting a specific cholesterol metabolic pathway to increase cytotoxic oxysterols Ming Lu1†, Xi-Han Hu1†, Qin Li1, Ying Xiong1, Guang-Jing Hu1, Jia-Jia Xu1, Xiao-Nan Zhao1, Xi-Xiao Wei1, Catherine C. Y. Chang2, Yin-Kun Liu3, Fa-Jun Nan4, Jia Li4, Ta-Yuan Chang2, Bao-Liang Song1* & Bo-Liang Li1* 1 State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 200031, China 2 Department of Biochemistry, Geisel School of Medicine at Dartmouth, Hanover, NH 03755, U.S.A. 3Liver Cancer Institute of Zhong Shan Hospital, Fudan University, Shanghai 200031, China 4National Center for Drug Screening, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China †These authors contributed equally to this work. *To whom correspondence should be addressed. E-mail: blli@sibs.ac.cn or blsong@sibs.ac.cn Liver plays central roles in cholesterol homeostasis. Hepatocellular carcinoma (HCC) impairs certain liver functions, and results in disorders in many processes including cholesterol metabolism. However, the link between disordered cholesterol metabolism and HCC development is unclear. Oxysterols are metabolites of cholesterol. The build up of unesterified oxysterols are cytotoxic to cells. Oxysterols produced and secreted by all extrahepatic tissues are further metabolized in liver. Here we report a HCC-specific cholesterol metabolic pathway that involves the induced ACAT2 gene expression, needed to esterify oxysterols for their secretion, in approximately 50% of advanced HCC. Inhibiting ACAT2 can specifically suppress the growth of HCC cell lines and xenograft tumors by increasing intracellular unesterified oxysterols. Further mechanistic studies reveal that HCC-linked promoter hypomethylation is essential for epigenetic induction of ACAT2 gene. Taken together, our findings imply that inhibiting ACAT2 can be used as a novel therapeutic strategy for HCC and other human cancer treatments. Identification of essential nucleotides in tRNALeu to its functions by using a constructed yeast tRNALeu knockout strain The fidelity of protein biosynthesis requires the aminoacylation of tRNA with its cognate amino acid catalyzed by aminoacyl-tRNA synthetase with high levels of accuracy and efficiency. Crucial bases in tRNALeu to aminoacylation or editing functions of leucyl-tRNA synthetase have been extensively studied mainly by in vitro methods. In the present study, we constructed two Saccharomyces cerevisiae tRNALeu knockout strains carrying deletions of the genes for tRNALeu(GAG) and tRNALeu(UAG). Disrupting the single gene encoding tRNALeu(GAG) had no phenotypic consequence when compared to the wild-type strain. While disrupting the three genes for tRNALeu(UAG) had a lethal effect on the yeast strain, indicating that tRNALeu(UAG) decoding capacity could not be compensated by another tRNALeu isoacceptor. Using the triple tRNA knockout strain and a randomly mutated library of tRNALeu(UAG), a selection to identify critical tRNALeu elements was performed. In this way, mutations inducing in vivo decreases of tRNA levels or aminoacylation or editing ability by leucyl-tRNA synthetase were identified. Full modified tRNALeu(UAG) WT and mutants were purified from yeast transformants and the in vitro experiments were performed. The results are identical with the in vivo ones. Overall, the data showed that the triple tRNA knockout strain is a suitable tool for in vivo studies and identification of essential nucleotides of the tRNA. Ablation of gp78 in liver improves hyperlipidemia and insulin resistance by inhibiting SREBP to decrease lipid biosynthesis Tong-Fei Liu1, Jing-Jie Tang1, Pei-Shan Li1, Yang Shen1, Jia-Gui Li§, Hong-Hua Miao1, Bo-Liang Li1, * and Bao-Liang Song 1, * 1 The State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, 320 Yue-Yang Road, Shanghai 200031, China. §Current address: Institut de Ge´ne´ tique et de Biologie Mole´ culaire et Cellulaire and Institut Clinique de la Souris, BP10142, 67404 Illkirch Cedex, France. Abstract gp78 is a membrane-anchored ubiquitin ligase mediating the degradation of HMG-CoA reductase (HMGCR) and Insig-1. As a rate-limiting enzyme in cholesterol biosynthesis, HMGCR undergoes rapid sterol-promoted degradation. In contrast, destruction of Insig-1 releases its inhibition on SREBP and stimulates the expression of lipogenic genes. Thus, gp78 has opposite effects on lipid biosynthesis. We here generated liver-specific gp78 knockout (L-gp78-/-) mice and showed that although the degradation of HMGCR was blunted, SREBP was suppressed due to the elevation of Insig-1/-2, and therefore the lipid biosynthesis was decreased. The L-gp78-/- mice were protected from diet- /age- induced obesity and glucose intolerance. The livers of L-gp78-/- mice produced more FGF21, which activated thermogenesis in brown adipocytes and enhanced energy expenditure. Together, the major function of gp78 in liver is regulating lipid biosynthesis through SREBP pathway. Ablation of gp78 decreases the lipid levels and increases FGF21, and is beneficial to patients with metabolic diseases. Regulation of pre-mRNA alternative splicing by a multifunctional protein YB-1 Shirong Mu, Wenjuan Wei, Monika Heiner, Lijuan Cao, Jingyi Hui The human Y box-binding protein 1 (YB-1) is a member of the evolutionarily conserved nucleic acid binding protein family, which exhibits multiple functions in the regulation of transcription, mRNA stability, and translation. It is mainly localized in the cytoplasm, but is highly expressed in the nucleus of tumors, particularly in breast cancer cells. A number of studies demonstrate its role in malignant transformation. Recently, several lines of evidence indicate that YB-1 is a spliceosome-associated protein and is involved in alternative splicing, but the underlying mechanism has remained elusive. In this study, we defined both CAUC and CACC as high-affinity binding motifs for YB-1 by SELEX and demonstrated that these newly defined motifs function as splicing enhancers. Interestingly, on the endogenous CD44 gene, YB-1 appears to mediate a network interaction to activate exon v5 inclusion via multiple CAUC motifs in both the alternative exon and its upstream polypyrimidine tract. U2AF65 is an essential splicing factor that recognizes the polypyrimidine tracts in the 3’ splice sites. Surprisingly, we found that U2AF65 does not bind to the polypyrimidine tract upstream of exon v5 directly. We provided evidence that YB-1 activates splicing by facilitating the recruitment of U2AF65 to weak polypyrimidine tracts through protein-protein interactions. In the mammalian genome, the sequences at the 3’ splice site are highly degenerate. A fundamental question about how such poor 3’ splice sites are recognized by the splicing machinery has remained unclear. Our findings suggest a new model for the recognition of weak 3’ splice sites. Caput epididymis-specific Cre transgenic mouse model and functions of miR-34a in spermatogenesis Shengsong Xie1, Juan Xu2, Jinxiong Han2, Xingxu Huang2*, Yonglian Zhang1,3* 1Shanghai Key Laboratory of Molecular Andrology, State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, 200031, People's Republic of China 2Model Animal Research Center of Nanjing University, Nanjing, 210061, People's Republic of China 3Shanghai Institute of Planned Parenthood Research, Shanghai, 200032, People's Republic of China *Correspondence ylzhang@sibs.ac.cn (Yonglian Zhang) or xingxuhuang@mail.nju.edu.cn (Xingxu Huang) To study the function of genes in the caput epididymis using Cre/loxP system, here, we reported that generated and characterized a constitutively active, principal cells of middle/distal caput epididymis-specific, Cre-expressing transgenic mouse line, which using the 1.8 kb fragment promoter of mouse Lcn5 gene. This transgenic mouse expression of Cre beginning in the late stages of postnatal epididymis development should be useful tool for future studies genes involved in the post-testicular sperm maturation in epididymis. Mammalian meiosis is unique to germ cells and a critical step in sexual reproduction. Essential for these events are programmed DNA double strand breaks (DSBs). By using bioinformatics method, miR-34a was predicted to target a number of genes which involve in DSB repair pathway, indicating that its potential functions in meiotic DSB. Using quantitative real time PCR assays, we identified that the miR-34a was preferentially expressed in the mouse testis, and their levels were up-regulated upon meiotic initiation during testicular development and in adult spermatogenesis. By generating germ cell specific overexpression mice model, we demonstrated that elevated miR-34a resulted in male infertility and meiotic arrest. Meanwhile, the expression levels of target genes which directly regulate meiotic DSB showed significant decline. Data will be presented to illustrate the gamatogenesis failure caused by meiotic DSB repair pathway disorder. Long noncoding RNAs with snoRNA ends We describe the discovery of sno-lncRNAs, a class of nuclear-enriched intron-derived long noncoding RNAs (lncRNAs) that are processed on both ends by the snoRNA machinery. During exonucleolytic trimming, the sequences between the snoRNAs are not degraded, leading to the accumulation of lncRNAs flanked by snoRNA sequences but lacking 5’ caps and 3’ poly(A) tails. Such RNAs are widely expressed in cells and tissues and can be produced by either box C/D or box H/ACA snoRNAs. Importantly, the genomic region encoding one abundant class of sno-lncRNAs (15q11-q13) is specifically deleted in Prader-Willi Syndrome (PWS). The PWS region sno-lncRNAs do not colocalize with nucleoli or Cajal bodies, but rather accumulate near their sites of synthesis. These sno-lncRNAs associate strongly with Fox-family splicing regulators and alter patterns of splicing. These results thus implicate a previously unannotated class of lncRNAs in the molecular pathogenesis of PWS. A Positive Role of Tet1 DNA Dioxygenase in the Regulation of AdultNeural Progenitor Cell Proliferation Abstract DNA hydroxylation catalyzed by Tetdioxygenases occurs abundantly in embryonic stem cells and neurons in mammals. However, its biological function in vivo is largely unknown. Here we demonstrate that Tet1 plays an important role in regulating neural progenitor cell proliferation in adult mouse brain. Mice lacking Tet1 exhibit impaired hippocampal neurogenesis associated with poor learning and memory. The mutant mice also exhibit DNA hypermethylation and down-regulation of genesrelated to the proliferation of neural progenitor cells in the adult hippocampus. Our results indicate that Tet1 is positively involved in the epigenetic regulation of neural progenitor cell proliferation in the adult brain. microRNA serves as a mRNA surveillance system by eliminating nonsense messages Ya Zhao, Yao Zhang, Xue Zhang and Ligang Wu State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, 320 Yueyang Road, Shanghai, China Abstract Eukaryotic cells are constantly at risk of various mutations, among which nonsense mutations are a severe type that will lead to pre-mature termination of translation, and result in the expression of potentially harmful C-terminal truncated proteins. Accordingly, the cells have evolved specialized mechanism, namely, nonsense mediated mRNA decay (NMD), to selectively eliminate aberrant transcripts harboring pre-mature stop codons (PTCs). One such remarkable mechanism is exon junction complex-mediated NMD (EJC-NMD), which depends on the recognition of a downstream EJC by translating ribosomes that stalled at PTC during pioneer round of translation. Although EJC-NMD is conserved among eukaryotes and can efficiently reduce the abundance of many targeted mRNAs, certain restrictions such as dependence of EJC and -50nt boundary rule limit its universility to all nonsense messages. MicroRNA (miRNA) is a class of small, non-coding RNA that previously known to inhibit translation and/or promote RNA degradation by imperfectly base-pairing with 3’UTR of target messages. In this study, we provide evidence that PTCs can potentiate miRNA mediated down-regulation of nonsense messages by translocating miRNA responsive elements (miREs) from ORF to 3’UTR upon PTC recognition. We show that APC (adenomatous polyposis coli), a tumor suppressor gene that is naturally subject to high frequency of nonsense mutations within last exon, which escapes EJC-NMD can nevertheless be repressed efficiently by miRNA induced NMD (miNMD). Additional experiments show that miNMD differs from EJC-NMD by employing RISC complex as effector proteins, substantial repression at translation level, as well as distinct boundary rule. We further searched for the PTC mutation in the mRNA and analyzed its relative abundance of HeLa cells and human samples by deep sequencing, and found additional messages that are specifically subject to miNMD regulation rather than EJC-NMD. These findings indicate that in addition to its role in tuning gene expression, miRNA may serve as an effective surveillance system to recognize and eliminate mRNAs bearing PTCs. Premature Termination Codons Are Recognized in the Nucleus in A Reading-Frame Dependent Manner Abstract mRNAs containing premature termination codons (PTCs) are known to be degraded via nonsense-mediated mRNA decay (NMD). Unexpectedly, we found that mRNAs containing any type of PTC (UAA, UAG, UGA) are detained in the nucleus whereas their wild-type counterparts are rapidly exported. This retention is strictly reading-frame dependent. Strikingly, our data indicate that translating ribosomes in the nucleus proofread the frame and detect the PTCs in the nucleus. Moreover, the shuttling NMD protein Upf1 specifically associates with PTC+ mRNA in the nucleus and is required for nuclear retention of PTC+ mRNA. Together, our data lead to a working model that PTCs are recognized in the nucleus by translating ribosomes, resulting in recruitment of Upf1, which in turn functions in nuclear retention of PTC+ mRNA. Nuclear PTC recognition adds a new layer of proofreading for mRNA and may be vital for ensuring the extraordinary fidelity required for protein production.