Hydrology

Hydrology Notes
E1.2E
E2.1C
Evaluate the future career and occupational prospects of science fields.
Explain, using specific examples, how a change in one system affects other Earth systems.
E2.3A
Explain how carbon exists in different forms such as limestone (rock), carbon dioxide (gas), carbonic acid (water), and animals (life) with Earth systems and how those forms can be beneficial or harmful to humans.
E2.3b
Explain why small amounts of some chemical forms may be beneficial for life but are poisonous in large quantities (e.g., dead zone in the Gulf of Mexico, Lake
Nyos in Africa, fluoride in drinking water).
E2.3c
E2.4B
Explain how the nitrogen cycle is part of the Earth system.
Explain how the impact of human activities on the environment (e.g. deforestation, air pollution, coral reef destruction) can be understood through the analysis of interactions between the four Earth Systems.
E4.1A
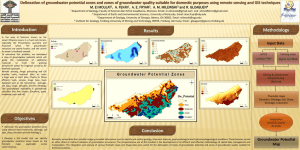
Compare and contrast surface water systems (lakes, rivers, streams, wetlands) and groundwater in regard to their relative sizes as Earth’s freshwater reservoirs and the dynamics of water movement (inputs and outputs, residence times, sustainability).
E4.1B
Explain the features and processes of groundwater systems and how the sustainability of North American aquifers has changed in recent history (e.g., the past 100 years) qualitatively using the concepts of recharge, residence time, inputs, and outputs.
E4.1C
Explain how water quality in both groundwater and surface systems is impacted by land use decisions.
E1.2E Careers
Hydrologist - scientist who studies the interactions between, quality of, and uses for groundwater and surface water systems.
Hydrogeologist - specifically studies groundwater
Meteorologist - scientist who studies the atmosphere.
E4.1A Surface Water Systems vs. Groundwater Systems
Water as a resource
75% of Earth is water
1% of that is groundwater
3% of Earth’s water is fresh water
30% of that fresh water comes from groundwater
>1% of that comes from surface water
69% comes from glaciers and ice
Surface water comes from precipitation and/or runoff (rain or snow) or bodies of water.
- Is easier to recharge (replenish) because it is closer to exposure of water source.
- primarily recharged by through precipitation and surface run-off
- also easier humans to discharge (for same reason)
- Easier to harvest, though in some cases not as “clean” as groundwater
- Has shorter residence time (time water stays in this reservoir before moving to a different location)
- Shorter sustainability (harder to sustain) due to exposure to more pollutants (land, air, and water)
- sustainability is more about quality (how good it is) than quantity (how much there is)
Groundwater comes from runoff and/or infiltration of precipitation (rain or snow) or bodies of water.
- Can lie a couple feet below the surface (such as in a marsh) or hundreds of feet below the surface such as in dry, arid regions of the West.
- The largest single supply of liquid fresh water available for use by humans (30% of all earth’s freshwater is stored underground)
* NOTE- ice locked in glaciers accounts for most, but not considered water
- More groundwater (estimated 1,000,000 cubic miles) in reserve as freshwater than surface water (30,300 mi 3 in world’s lakes and streams)
- Only a fraction of this groundwater can be tapped by wells and springs
- Harder to recharge (and discharge )
- Has longer residence time (time water stays in this reservoir before moving to a different location)
- Longer sustainability (easier to sustain) because it is not exposed to as many pollutants
- Aquifers can be recharged (replenished):
1. naturally by precipitation and runoff a. deeper it is, the longer it takes to replenish b. drier areas take longer to replenish
2. artificially a. water spreading (via dams, ditches, furrows, or pits) b. recharge wells where water is directly recharged into aquifer (more expensive)
- The larger the pores (high porosity) or fractures in rock, the more water can be transmitted or permeated through (greater permeability). Thus, some rocks are better aquifers than others.
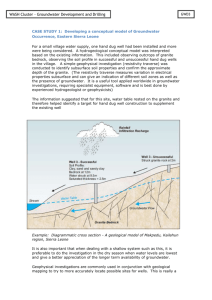
E4.1B Groundwater Systems and Aquifer Reliability
Aquifers - can store and transmit water underground
- classified as confined (layer of impermeable rock lies above) or unconfined
- People are more reliant on aquifers now for freshwater source (supply and demand)
Wells - can discharge groundwater for human use. Wells facilitate the movement of groundwater from the aquifer to the surface.
*
How they’ve changed in past 100 years (sustainability)
- Growth of industry, population, technology, and water use has put stress on land and water resources. Locally, water quality has degraded in recent years, due to city and
industrial wastes as well as chemical herbicides, pesticides, and fertilizers from agricultural use. Other causes are sewer and septic leaks, landfill leak percolation, and salt water infiltration from excessive groundwater pumping near coastal regions.
Recharge (inputs)
- Aquifers can be recharged (replenished):
1. naturally by precipitation and runoff a. deeper it is, the longer it takes to replenish b. drier areas take longer to replenish
2. artificially a. water spreading (via dams, ditches, furrows, or pits) b. recharge wells where water is directly recharged into aquifer (more expensive)
Discharge (outputs)
- use by plants ( evapotranspiration )
- wells and springs (and drains)
- human extraction
- Ogallala aquifer (KS, AR, TX) used for irrigation
- underflow discharge (moving from one place in an aquifer to another)
Residence time
- Shorter residence time because people are relying more on groundwater use. Making more deposits from groundwater , so groundwater doesn’t sit in aquifer for as long as it used to 100 years ago.
- This can cause sinkholes if too much groundwater is being pumped.
E4.1C Water Quality Impacted by Land-Use Decisions
- Growth of industry, population, technology, and water use has put stress on land and water resources. Locally, water quality has degraded in recent years, due to city and industrial wastes (underground tank leaks) as well as chemical herbicides, pesticides, and fertilizers from agricultural use. Other causes are sewer and septic leaks, landfill leak percolation , and salt water infiltration from excessive groundwater pumping near coastal regions.
- wetlands have played a valuable role in water quality, as they act as a natural filter and protective reservoir for water pollutants and contaminants.
- As population and urbanization continues to grow, chances for groundwater contamination increases.
- As water moves across land (mainly farm land or livestock area), it picks up loose soil, manure, fertilizer, and pesticides, which can affect water quality. Water can also be polluted indirectly by air pollution (acid rain).
- Earth is considered a closed system, where all parts affect each other. A change in one of the Earth’s systems can alter another system.
(E2.1C)
EX: Factories emit sulfur and carbon dioxide into the atmosphere, which mixes with atmospheric gases to form acidic concentrations when mixed with water.
This change in the atmospheric system can affect the local water systems.
- Saltwater intrusion- Saltwater (from oceans and salt lakes) can move underground into groundwater systems. Since saline (salty) water is denser than freshwater, it sinks to the bottom. When groundwater is discharged, however, the demarcation of freshwater and saltwater is mixed and this can pollute the groundwater.
- Mainly a problem in CA, FL, NJ, and SC (all coastal states)
- Dissolved Oxygen levels drop as water temperature increases
Nitrogen Involved (E2.3c) (E2.3b)
- Sources of Nitrogen
Natural: atmosphere (biggest source) lightning, fires, volcanic eruptions, dead animals, nitrogen-fixing bacteria
Human: burning fossil fuels, burning lumber, fertilizers, farming, sewage waste
- The addition of Nitrogen (from combustion of fossil fuels and biologically from animals) to the earth system of cycles is causing acid rain (Nitric Acid) and this is polluting surface water and groundwater systems. Nitrogen combines chemically with atmospheric oxygen and when mixed with water, becomes a contaminant. (E2.3c)
(E2.4B)
- Burning fossil fuels produces primarily carbon dioxide, nitrogen oxide, and sulfur dioxide.
- Nitrogen is needed for nutrients. Aquatic animals eat (absorb) the nitrogen and convert it into a waste (ammonia- NH
3
or carbon dioxide- CO
2
). This waste then is converted and cleansed by certain bacteria into harmless, useful forms that can be used again by plants.
This is known as the Nitrogen Cycle.
- Nitrogen can also be washed away (run-off) into large river systems. These river systems can eventually wash all this accumulated nitrogen into larger bodies of water. An excessive amount nitrogen increases the amount of aquatic plant life that grows and this lowers the amount of dissolved oxygen (eutrophication). These conditions (such as the
“dead zone” in the Gulf of Mexico) can kill off aquatic life, and prevent further life from existing. This can impact the economy as well as the food chain. (E2.3b) (Glencoe pgs.
229-230)
- Lake Nyos (Africa)- a resurgent volcano lying beneath the lake degasses and emits CO
2 into the cold, bottom portion of the lake. The CO
2
stays trapped near the bottom due to thermal stratification. When the water at the bottom is disturbed, the CO
2
rises to the top and is emitted from the lake. This abundance of CO
2
into the atmosphere is too much for human beings and animals to breath and becomes deadly. In 1986, nearly 1700 people and over 3000 cows died when an “avalanche” of CO2 gas spread through the nearby areas. (E2.3b)
- Too much fluoride in your drinking water can be hazardous to teeth and bones, as the excessive fluoride weakens tooth enamel and weakens bones, making fractures or breaks more common. The recommended amount is anywhere from 2 to 4 milligrams per Liter.
(E2.3b)
- State- and Federal-mandated analyses are done regularly to try and control/prevent these pollutants from degrading the fresh groundwater supply.
- Bigger demand for fresh groundwater in the last 100 years than ever before.
Therefore, efforts are greater to protect it.
- For the most part, groundwater is acceptable for most uses.
- Groundwater used for: a.
Domestic Use (households) b.
Industrial (business and machines) c.
Agricultural (farming) d.
Hydrothermal/Geothermal Energy (heating using water)
- Layers of soil and rock act as natural filters of bacteria as water percolates through.
- Planting vegetation allows for more filtration. Paving over vegetation and rocks and soil means less filtration and more run-off. This lowers groundwater quality and quantity in a given area.
- Sometimes, water picks up too many dissolved minerals and can be too salty for humans, animals, and plants.
- Hard water = water that contains a lot of calcium and magnesium
- Soft Water = water that contains little calcium and magnesium
- Soft water used for laundry, dishwashing, and bathing
- Acidic water (pH less than 7) can rust pipes and stain clothing
- Domestic water should have pH between 5.5 and 9
What has the Government Done?
- Clean Water Act – a 1977 amendment to the Federal Water Pollution Control Act of 1972 that regulates discharges of contaminants into the waters of the United States.
- Safe Drinking Water Act – a 1974 law established to protect the quality of drinking water in the
United States.
- Resource Conservation and Recovery Act – a 1976 law that gave the EPA the authority to control hazardous waste from the “cradle to grave”.
- Comprehensive Environmental Response, Compensation, and Liability Act – a 1980 law that provides Federal funding to help clean up uncontrolled or abandoned hazardous waste sites as well as accidents, spills, and other emergencies. Referred to as the “Superfund”. The government can force companies to pay to clean up their mess.
E4.2B Explain how the impact of human activities on the environment (e.g. deforestation, air pollution, coral reef destruction) can be understood through the analysis of interactions between the four Earth Systems.
Burning fossil fuels and using fertilizers that contain nitrogen produces excess amounts of nitrogen oxide and ammonia gas into the atmosphere. These are converted into nitric acid and returns to the earth as acid rain, which damages surface water, plants, and soil.
Burning oil and coal can also release sulfur dioxide, which turns into sulfuric acid and falls as acid rain as well, causing the same damages described above.
Also toxic metals like lead, cadmium, and arsenic can produce air pollution.
Interaction between geosphere (fossil fuels produced inside earth), atmosphere (toxic gases emitted into the air and converted and transported), hydrosphere (toxic gases converted into acid precipitation) and biosphere (plants damaged or destroyed).
How does this affect me? Why should I care?
- When ground is not porous enough, floods can occur
- Most of you rely on groundwater (over half of the state of Michigan) for your domestic needs
(drinking water, bath water, laundry and dishwashing water, irrigation water)
- Groundwater is a big source of freshwater reserve.
- Most of the bottled water you drink comes from groundwater
(EX: Absopure, Mountain Valley Spring Water, etc.)
- If you are a farmer , you rely heavily on groundwater and your actions/management of your farm greatly impacts the quality and supply of groundwater
Hydrogeology Sources:
Michigan Department of Education: High School Science Content Expectations/Earth
Science
Careers in Hydrogeology (E1.2E) http://www.allen-york.com/?gclid=CJaTmcTo5YwCFRUHWAod4TDB7Q
Hydrology Notes http://74.125.95.132/search?q=cache:Lylo8qUpuCUJ:pioneer2.aaps.k12.mi.us/hentz/PowerPoint s/Hydrogeology.ppt+groundwater+surface+water+E4.1A&cd=1&hl=en&ct=clnk&gl=us
National Atlas of the United States- Plot Data for weather, watersheds, aquifers, rivers, etc.) http://www.nationalatlas.gov/natlas/Natlasstart.asp
Purdue University’s Hydrogeology Web Page http://www.purdue.edu/dp/envirosoft/groundwater/src/geo.htm
Hydrology Animations Web Site http://www.educypedia.be/education/hydrology.htm
Freshwater Reservoirs Numbers http://ga.water.usgs.gov/edu/watercyclefreshstorage.html
USGS- Water Resources of the United States http://water.usgs.gov/ http://water.usgs.gov/education.html
http://ga.water.usgs.gov/edu/mearth.html
Water Cycle (E4.p1A)
Water Cycle Diagram http://ga.water.usgs.gov/edu/watercyclehi.html
Water Cycle Animation - VERY GOOD http://www.scwa.com/flash/water_cycle_rev_1.swf
Water Cycle Animation - good for runoff and infiltration http://www.region.waterloo.on.ca/web/region.nsf/0D78CB956F92D4BB85256C6B005A62C7/$ file/hydrologic2.swf?openelement
Water Cycle Breakdown and Animation http://ww2010.atmos.uiuc.edu/(Gh)/guides/mtr/hyd/home.rxml
Water Cycle Animation http://observe.arc.nasa.gov/nasa/earth/hydrocycle/hydro1.html
- Transpiration http://piru.alexandria.ucsb.edu/collections/geography3b/misc/transpiration%5b1%5d.jpg
- Evaporation http://www.sites.fse.ulaval.ca/tictheque/sites/cycleeau02/evaporation%20image.gif
- Condensation http://www.2xup-ph.org/album/discovery/condensation.jpg
(animation) http://www.earthsci.org/weather/weaimages/cndnsate.gif
- Precipitation http://ellerbruch.nmu.edu/classes/cs255f02/cs255students/abarker/P4/precipitation.html
- Surface Runoff http://imnh.isu.edu/digitalatlas/hydr/basics/main/imgs/runoff.jpg
- (Stream runoff picture) http://esm1.versar.com/PPRP/potomac/Transect_Photos/T182-Runoff_from_Fairfax_Intake_Construction.jpg
Watershed (E4.p1B)
Animated Watershed http://www.co.berks.pa.us/conservation/lib/conservation/images/watershed/what_is_watershed_a ction.gif
Watershed Functions and Processes http://danr.ucop.edu/uccelr/h04.htm
EPA Watershed info http://www.epa.gov/owow/watershed/whatis.html
Find Your Watershed http://www.epa.gov/owow/watershed/region/
Clinton River Watershed http://cfpub.epa.gov/surf/huc.cfm?huc_code=04090003 http://www.crwc.org/watershed/whatiscrwc.html
Detroit River Water Watershed http://cfpub.epa.gov/surf/huc.cfm?huc_code=04090004
Streams and Rivers (E4.p1C)
Online River Lab http://www.sciencecourseware.org/VirtualRiver/Flooding/index.html
Impact of Human Land Use Decisions http://www.boquetriver.org/adopthumanimpact.html
http://regentsprep.org/Regents/global/themes/geography/imp.cfm
Wetlands (E4.p1D) http://www.epa.gov/wetlands/ http://www.water.ncsu.edu/watershedss/info/wetlands/funval.html
Surface Water Ground Water Interactions http://water.usgs.gov/ogw/gwsw.html
Groundwater
Michigan Groundwater http://gwmapinfo.rsgis.msu.edu/
Pics of Confined/Unconfined Aquifers http://www.purdue.edu/dp/envirosoft/groundwater/src/geo3.htm
Michigan tech Groundwater Info and Images http://techalive.mtu.edu/meec/module04/sitemap.htm
Groundwater- Good info and graphics http://www.groundwater.org/gi/gi.html
Interactive Map of Michigan Relating to Groundwater http://gwmap.rsgis.msu.edu/viewer.htm
Interactive Reports of Michigan Relating to Groundwater http://gwmapinfo.rsgis.msu.edu/
Groundwater Recharge and Input (E4.1A) http://www.kgs.ku.edu/Publications/Bulletins/ED10/07_manage.html
Groundwater vs. Surface Water Amount Graphic http://capp.water.usgs.gov/GIP/gw_gip/compar.html
San Diego State Groundwater Page http://groundwater.sdsu.edu/
Groundwater Use in the U.S.- Good with charts and graphs and trends http://ga.water.usgs.gov/edu/wugw.html
How Groundwater Works http://academic.evergreen.edu/g/grossmaz/KIEPERME/
USGS- Groundwater Storage http://ga.water.usgs.gov/edu/watercyclegwstorage.html
Groundwater Storage (labeled pic) http://imnh.isu.edu/digitalatlas/hydr/concepts/gwater/imgs/6comp.jpg
USGS- Groundwater (with images) http://capp.water.usgs.gov/GIP/gw_gip/gw_a.html
USGS- Groundwater diagram http://capp.water.usgs.gov/GIP/gw_gip/how_occurs.html
Groundwater Quality (Watersheds)- Includes THREE VIDEOS on watersheds http://www.conservationinformation.org/?action=learningcenter_kyw_whatisawatershed http://capp.water.usgs.gov/GIP/gw_gip/quality.html
Groundwater/Aquifer Water by Use Graph/Chart http://www.edwardsaquifer.net/images/uses.gif
Discharge and Residence Time of Ground Water in the Chesapeake Bay Watershed http://md.water.usgs.gov/publications/fs-150-99/html/index.htm
Safe Drinking Water Act- Helpful Map/Diagram http://www.epa.gov/safewater/publicoutreach/images/landscape_1200x776.jpg
Bottled Water Drilling Pic http://www.fda.gov/fdac/graphics/2002graphics/water_diag.jpg
Types of (Aquifer) Wells http://ga.water.usgs.gov/edu/earthgwwells.html
Impact of Land Use on Groundwater Quality
Abstract on how land use has affected Michigan’s groundwater use http://pubs.usgs.gov/of/2005/1269/
Abstract on the increase in levels of various elements in MI groundwater http://mi.water.usgs.gov/pubs/WRIR/WRIR00-4120/
Article on Effect of Rapid Residential Development near Higgins Lake http://mi.water.usgs.gov/splan3/sp00398/higginslk.php
Residence Time
Groundwater Residence Time http://sofia.usgs.gov/publications/sir/2004-5069/
Overall Residence Time http://maps.grida.no/library/files/world_s_water_cycle_schematic_and_residence_time.jpg
Surface Water
USGS with charts and tables useful for info http://ga.water.usgs.gov/edu/wusw.html
Watersheds and Karst Topography
Karst Topography with pics of features http://www.watersheds.org/blue/earth/karst.htm
Good explanation of the watershed and components http://www.watersheds.org/ http://www.watersheds.org/earth/hydrology.htm
Drainage Basins
Drainage Basin with graphics http://www.physicalgeography.net/fundamentals/10aa.html
Drainage Patterns with graphics (as well as other ESC links) http://www.il-st-acad-sci.org/kingdom/geo1005.html
Sustainability
Steps to Surface Water and Groundwater Sustainability http://www.swfwmd.state.fl.us/about/isspapers/watersupply.html
Good Article on Aquifer Sustainability (by a write from Mississippi) http://www.msfb.com/news/Farmcountry/march04/Aquifer%20sustainability.html
Water Quality
Sources of Groundwater Contamination http://www.groundwater.org/gi/sourcesofgwcontam.html
GOOD PIC of Groundwater Contamination from Urbanization http://www.livinghistoryfarm.org/farminginthe40s/media/water_1201.gif
GOOD PIC of how Groundwater can get contaminated miles from its original source through aquifer percolation http://www.gibsons.ca/gibsonswater/unjimageswater/imagesdiagrams/groundwatergibsons.gif
Groundwater and its contamination http://edugreen.teri.res.in/explore/water/health.htm
Saltwater Intrusion Pic http://pubs.usgs.gov/gip/gw/intrusion.html
Activities that cause Groundwater contamination http://wse20.deh.ehnr.state.nc.us/swap/pages/whpactiv.htm
Water Quality and Wells (Michigan-related) http://web1.msue.msu.edu/waterqual/docs/wq02p1.html
Water Quality Info and Action http://www.epa.gov/safewater/dwinfo/mi.htm
Water Quality and Land Use https://engineering.purdue.edu/SafeWater/watershed/landuse.html#table1
Bottled Water http://www.bottledwater.org/
Use of Water in the United States http://water.usgs.gov/watuse/
Water Usage Games/Quizzes http://www.gem.msu.edu/gw/educ.html
Michigan Counties http://www-personal.umich.edu/~bbowman/birds/mich_co.html
Carbon in Earth Systems (E2.3A)
Common Acids http://hyperphysics.phy-astr.gsu.edu/Hbase/Chemical/acidcom.html
The Dead Zone (E2.3b) http://serc.carleton.edu/microbelife/topics/deadzone/ http://www.smm.org/deadzone/
Nitrogen in Water http://www.lenntech.com/elements-and-water/nitrogen-and-water.htm
Lake Nyos (E2.3b) http://pagesperso-orange.fr/mhalb/nyos/nyos.htm
http://www.geo.arizona.edu/geo5xx/geos577/projects/kayzar/html/lake_nyos_disaster.html
http://www.hprcc.unl.edu/nebraska/Nyos.html
Too much Fluoride in your Drinking Water (E2.3b)
Study of its effects http://sci.tech-archive.net/Archive/sci.med.dentistry/2006-03/msg00619.html
Article on Too Much Fluoride http://www.surfersam.com/articles/fluoride-drinking-water.htm
The Nitrogen Cycle (E2.3c) http://www.windows.ucar.edu/tour/link=/earth/Life/nitrogen_cycle.html&edu=high http://www.physicalgeography.net/fundamentals/9s.html
http://www.ucar.edu/news/backgrounders/nitrogen.shtml
http://www.aquarticles.com/articles/management/Nitrogen%20Cycle.jpg
http://soil.gsfc.nasa.gov/NFTG/nitrocyc.htm
Nitric Acid http://www.elmhurst.edu/~chm/vchembook/193nox.html
http://www.texasep.org/assets/images/gwcont.GIF