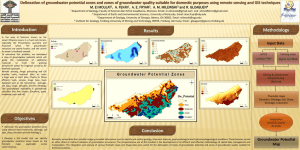
Hydrogeochemical Characteristics of Groundwater Surrounding
advertisement

Hydrogeochemical Characteristics of Groundwater Surrounding Gold Mine Area in Phichit - Phetchabun Provinces Jirawan Thamrongsrisakul(1), Srilert Chotpantarat(2,3) (1) Inter-department of Environmental Science, Graduate School, Chulalongkorn University Bangkok,10330, Thailand. (2) Department of Geology Faculty of Science Chulalongkorn University, Bangkok, 10330, Thailand. (3) Center of Excellence on Hazardous Substance Management (HSM) , Chulalongkorn University, Bangkok, Thailand * E-mail:Jirawan_t@hotmail.com, csrilert@gmail.com Abstract : In last decade, groundwater is an important source of water. This study focused on groundwater quality located about 280 km. north from Bangkok on the border between Phichit and Phetchabun Provinces. Due to the current economic expansion has increased and in turn impact on groundwater quality. Since there are a variety of geological features and human activities, including mining and agricultural community in such area, the correlation technique of hydrogeochemical parameters would be considered to address the sources of groundwater contamination. This study samples were collected 47 groundwater wells in May 2013(summer) with a different geological characteristics and land use types. There are 4 layers which are weathered or fractured volcanic rock, rhyolite and andesitic tuff (Vw) of 53.19%, volcanic rock, rhyolite and andesitic tuff (Vf) of 23.40%, floodplain deposit and clay dominant (Qfd) of 12.47%. and hard volcanic rock, andesitic and diorite (Vm) of 10.64%. According to Piper diagrams, the results showed that groundwater chemistry was Groundwater types and the percentages for each type were: Ca-HCO3 (53.19%), Ca-Mg-Cl (14.89%), Ca-Na-HCO3 (8.51%), Ca-Cl (10.64%), Na-Cl (8.51%), Na-HCO3 (4.26%). This study Furthermore, the correlation matrix, implemented the correlation matrix among the chemical constituents, showed highly relationships as the descending order: TDS-EC (r = 0.906), As-Pb (r=0.771) ,Fe-Zn(r=0.622) and Mg-SO42-( r =0.812). Mg2+ in groundwater often comes from the input of the dissolution of dolomite and sulfate (SO4 2−) may come from rainfall and agrochemical fertilizers. Soil was controlled by weathering rocks in area and ZnPb may occur from oxidation product of primary lead sulfide ore (galena,PbS), (Sphalerite, ZnS) and Arsenopyrite, an iron arsenic sulfide (FeAsS, FeS2, FeAs). In summary, both natural and human activities may affect groundwater quality around such gold mine area. Keywords:Groundwater, Hydrogeochemical characteristics, statistics, Gold Mine Introduction Groundwater contamination is a major problem for a long term period. Groundwater quality in a region may be affected by the natural processes and/or anthropogenic activities (Jiang et al.2009). Groundwater may be contaminated by leaching process of chemicals in the soil surface and finally reach into underneath aquifers. In this area, there have been mining processes used of chemicals in the gold extraction process and in turn may accelerate the natural processes and result in groundwater contamination in Figure.1 Location of the study area and groundwater sampling sites. higher doses (Conesa et at., 2007). Under acid mine drainage, heavy metals may release and finally cause contamination of surface water and groundwater during mining operation or even long after mine closure (Changul et al.2009). Since most land use are agricultural areas along with the use of the excessive agrochemicals, many studies found that concentration of nitrate and sulfate in groundwater monitored higher than those found in the past (Aravena et al., 1999; Compton and Boone, 2000; Jiang et al., 2008). This study used statistical technique, correlation analysis, to identify the cause of groundwater contamination (Reimann et at., 2002; Farnham et at., 2003, Ouyang, 2005; Lin et at., 2012), Although chemical compositions of groundwater explained groundwater quality, however, it is not easy to distinguish the contributions from natural weathering processes and anthropogenic inputs based on the chemical composition of groundwater alone. Thue, such technique help to interpret the relationship between hydro geochemical properties and sources of groundwater contaminatino (Chan, 2001). The purpose of this study was to use statistical methods to distinguish the impact of natural and anthropogenic processes affecting on groundwater quality around this gold mine area. 2. Description of study area water was the sole drinking and irrigation sources for local residents. Study area (Figure.1) is located in Phichit and Phetchabun provinces and covered total area approx. 504 km2. The geological characteristics of the study area generally are fluvial deposits (73.3%), terrace deposits (22.6%), rhyolite (0.83%) and andesite (3.29%). There are 7 land use types and the percentages for each type were: 69.2 % of rice growing land, 12.44% of integrated agriculture, 6.24% of Eucalyptus, 5.74% of deciduous, 4.54% of construction land, 1.02% of Mining and 0.79% of water. 3. Methodology 3.1. Groundwater level measurements A groundwater level survey was performed at the site during May 2013 (summer season). Groundwater levels in the wells (n = 47) were determined manually using a water level meter. Groundwater elevations (m, asl.) were calculated in a GIS platform by subtracting depth to water measurements from the topographic elevations obtained from the digital elevation model (DEM) of the site. Then groundwater elevation values were contoured using the ordinary kriging spatial interpolation method available in the Geostatistical Analyst extension of the ArcGIS 9.3 software (ESRI, 2009). Additionally, for comparison purpose, historic groundwater levels were digitized from an earlier study conducted in the area. 3.2. Studied sites fourty seven groundwater samples were collected from all 47 springs from the system (Figure.1). 11 samples were collected from the fluvial deposits and rice, 2 from terrace deposits and rice, 19 from fluvial deposits and construction land, 9 from terrace deposits and construction land, 1 from rhyolite and construction land, 3 from fluvial deposits and integrated agriculture, 2 from terrace deposits and integrated agriculture. It is worth emphasizing that 3.3. Sampling and analytical procedures Groundwater samples were collected in May of 2013. The water temperature (T), pH, electrical conductance (EC), Total Dissolved Solids (TDS), oxidation reduction potential (ORP) and salt were measured by a hand-held water quality meter in field. In addition 1 L sample were collected in plastic containers and stored at 4 °C and analyses at laboratory of the Interdepartment of Environmental Science laboratory at Chulalongkorn University. The concentrations of SO2-4, PO3−4 , NO-3 were determined using UV-VIS spectrophotometer. Chorine ion, HCO-3 and CN- were determined in the laboratory by volumetric titration. Major cations (Mg2+ ,K+, Na+, Ca+) and trace elements (Cr6+, Fe, Mn, Ni, As, Al, Co, Cu, Pb and Zn) were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS) following the US Environmental Protection Agency (EPA) standard methods (APPHA, AWWA and WEF., 2005)(Table 1) 3.4. Statistical analysis The correlation matrix among the chemical constituents for the groundwater samples carried out based on the data matrix of the chemical parameters for groundwater samples. The variables for correlation analysis were T, pH ,EC, TDS,ORP, Salt, SO2-4, PO3−4, NO-3, Cl-, CO32-,HCO-3 ,CN-, Mg2+ , K+, Na+, Ca+, Cr6+ , Fe, Mn, Ni, As, Al, Co, Cu, Pb and Zn. Table 1 Chemical analyses of the groundwater. well X 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 670242 671640 675901 681665 679216 674666 667682 665682 668187 668182 672819 671963 671470 674182 675665 676132 674688 674468 672074 667095 675364 677014 679580 679977 682127 681777 683938 685906 685204 686128 685984 680626 676971 676090 669061 668841 667715 666139 667202 670571 672068 673800 677457 679611 679005 678393 673213 Y 1813416 1811482 1813194 1815676 1815398 1817458 1815659 1803469 1797786 1797762 1797504 1799053 1799131 1802308 1804261 1806312 1808852 1807172 1806623 1807299 1796163 1796959 1800189 1800294 1803150 1803413 1803989 1802290 1805119 1807416 1809078 1807966 1797543 1798763 1803521 1804870 1806228 1815675 1815564 1815079 1815753 1816170 1816107 1808991 1808460 1805734 1802321 Mean Min MaX SD CV Z 50 49 70 89 67 59 55 53 48 41 69 49 55 84 71 70 69 71 75 55 60 73 75 73 88 86 87 85 85 95 106 83 87 78 53 62 61 46 58 51 56 56 72 70 78 78 99 T pH ORP EC TDS Salt + CO3 2- HCO3 - K 30 6.45 -20.5 410 274 204 0 190 31.2 7.87 114.6 1075 720 537 0 388 33.6 7.01 140.4 503 336 251 0 215 28.7 6.67 -69.3 503 338 252 0 160 37.5 7.48 177.3 8.13 546 4.08 0 303 30 7.19 113.7 487 326 244 0 235 36.3 8.85 29 529 355 264 15 240 29.5 6 93.5 175.3 117.5 87.7 0 83 29.7 6.71 118.7 1117 747 558 0 459 30.2 6.73 48.9 752 504 376 0 325 37.9 7.4 125.7 997 667 498 0 364 33.7 7.02 1.2 617 413 308 0 274 38 7.3 65.6 718 481 359 0 286 29.6 7.48 -59.6 407 272 203 0 190 47.5 6.73 -146.8 603 404 301 0 180 29.7 7.09 62.7 730 489 365 0 230 35.3 7.44 91.8 7.35 492 368 0 300 29.6 6.94 -16.6 972 649 484 0 424 30.6 6.44 -1.7 297 199 149 0 131 30.6 7.4 55.2 116 781 583 0 570 29.5 7.15 124.3 628 420 314 0 267 29.9 6.76 -38.3 436 295 218 0 257 31.6 7.91 78 834 558 417 0 352 30.2 7.26 -101.7 1090 729 544 0 476 29.8 7.09 28 1123 750 562 0 404 31 6.76 84.5 1581 1059 790 0 456 29.1 7.01 -174.9 775 518 387 0 200 30.9 7.2 -100.8 930 623 465 0 403 30.7 7.34 85.5 729 488 364 0 273 29 7.03 -101.1 766 513 383 0 265 47.7 7.1 119.8 477 320 239 0 156 29.1 6.98 -37.7 632 423 316 0 251 29.5 6.7 47 375 251 187 0 149 35.7 6.78 92.7 2700 1800 1350 0 275 30.2 7.23 172.7 286 192 143 0 124 30.2 7.58 146.6 1271 852 636 0 1140 29.3 7.39 163.7 1321 885 660 0 656 30 8.12 0.6 739 495 369 22 255 29.5 8.07 98.5 602 404 301 15 263 29 7.05 -37.2 484 324 242 0 2220 36.5 7.15 123 780 522 390 0 354 28.7 7.81 38.8 538 360 269 0 255 29.2 6.58 -47.9 277 184 139 0 165 32.6 6.84 -176.4 2150 1440 1080 0 257 29.5 6.91 1.2 1621 1089 813 0 490 31.2 7.23 93.3 468 216 235 0 184 29.7 7.39 -100.9 433 289 216 0 227 31.883 7.1621 32.023 746.17 534.24 392.02 1.1064 347.26 28.7 6 -176.4 7.35 117.5 4.08 0 83 47.7 8.85 177.3 2700 1800 1350 22 2220 4.2481 0.4977 93.883 513.05 323.55 247.87 4.365 328.03 0.1332 0.0695 2.9317 0.6876 0.6056 0.6323 3.9453 0.9446 0.580 1.377 0.509 0.887 1.995 1.771 1.714 1.044 0.484 0.414 0.564 0.764 0.670 1.405 1.870 0.588 0.776 0.461 0.380 0.893 0.608 1.646 0.578 0.789 0.897 1.142 0.464 1.382 1.070 1.296 1.834 0.603 1.220 0.397 0.688 1.043 0.620 1.406 0.868 0.476 1.189 0.763 0.398 1.031 1.194 0.807 0.972 0.947 0.380 1.995 0.447 0.471 Mn 0.365 0.490 0.490 0.435 0.250 0.500 0.430 0.620 0.358 0.307 0.342 0.502 0.409 0.745 0.420 0.435 0.492 0.592 0.517 0.562 0.533 0.537 0.549 0.750 0.709 0.699 4.060 0.949 0.981 0.864 0.893 0.917 2.775 0.720 0.713 0.788 0.725 0.736 0.767 0.900 0.799 0.945 0.913 1.083 1.025 0.788 1.040 0.775 0.250 4.060 0.618 0.798 Mg 2+ 25.042 52.085 56.599 26.127 31.623 10.561 15.661 11.689 23.767 6.032 32.708 12.964 6.457 6.911 12.100 25.335 14.313 33.177 3.994 12.671 14.078 11.147 23.914 36.167 24.104 38.454 24.588 10.781 23.488 20.689 16.336 19.399 17.508 68.897 7.658 21.979 24.045 18.871 38.351 9.842 7.512 10.605 17.699 76.416 96.922 18.608 12.510 24.051 3.994 96.922 19.027 0.791 NO-3 2.415 1.267 2.336 4.038 2.177 0.990 0.204 2.893 2.173 0.419 0.114 0.226 2.742 0.732 1.637 0.356 2.588 1.674 1.905 0.682 1.498 2.319 1.689 0.284 8.832 1.742 3.995 1.087 1.247 0.641 1.358 2.227 1.148 2.441 0.502 1.251 1.161 0.318 1.596 0.898 1.763 1.791 0.449 3.335 1.687 0.263 1.045 1.662 0.114 8.832 1.444 0.869 SO2-4 29.180 63.708 55.843 48.531 33.569 7.251 18.829 24.377 44.272 25.566 19.581 21.714 4.120 3.767 13.688 36.738 15.959 14.571 8.141 5.785 23.348 47.618 27.223 41.449 40.889 50.518 15.660 13.788 30.822 46.666 20.709 42.607 22.842 77.666 15.668 16.550 20.118 25.880 15.553 11.456 16.052 11.801 20.563 77.577 77.224 42.047 15.737 29.005 3.767 77.666 19.346 0.667 PO3− 4 Cl 0.221 0.051 0.204 0.148 0.014 0.177 0.026 0.197 0.016 0.025 0.015 0.162 0.018 0.241 0.011 0.011 0.011 0.011 0.026 0.010 0.005 0.113 0.010 0.013 0.014 0.027 0.148 0.027 0.101 0.034 0.014 0.034 0.008 0.025 0.054 0.008 0.007 0.006 0.013 0.071 0.014 0.091 0.244 0.014 0.006 0.019 0.118 0.060 0.005 0.244 0.073 1.205 76.476 59.981 79.475 246.424 66.979 73.977 99.469 51.484 62.980 246.424 52.984 38.488 70.978 105.967 623.307 71.478 326.399 21.493 578.821 24.492 69.978 246.424 29.491 727.774 64.980 66.479 46.486 66.479 80.475 73.477 281.413 378.383 271.416 62.481 246.424 23.993 153.952 38.988 396.377 281.413 421.369 71.478 386.380 63.980 183.943 93.971 58.982 167.342 21.493 727.774 170.200 1.017 Na+ 39.220 74.220 316.900 17.880 192.900 69.350 10.310 61.640 311.200 280.100 5.737 24.260 3.261 11.430 18.880 3.090 19.370 1.070 54.660 24.000 85.050 11.170 20.420 38.040 56.350 27.200 53.890 128.400 10.120 32.320 58.410 13.640 51.320 48.000 32.140 19.900 4.073 3.799 4.397 5.820 43.720 20.830 14.160 5.337 24.690 34.370 20.620 51.227 1.070 316.900 75.075 1.466 Al Ca Cr6+ Fe Co 0.016 20.580 0.002 1.072 0.002 0.013 38.330 0.002 0.364 0.001 0.009 2.510 0.002 9.642 0.001 0.013 16.360 0.002 2.973 0.002 0.021 32.330 0.002 0.093 0.001 0.009 29.330 0.002 4.965 0.001 0.019 8.537 0.002 0.133 0.001 0.705 4.891 0.002 2.770 0.002 5.022 26.920 0.002 0.066 0.001 0.013 23.050 0.002 0.073 0.001 0.015 20.240 0.002 0.224 0.002 0.018 24.240 0.002 0.123 0.001 0.010 1.970 0.002 0.103 0.001 0.007 8.500 0.002 0.326 0.001 0.007 16.700 0.002 0.075 0.001 0.006 18.140 0.002 0.060 0.001 0.001 23.940 0.002 0.053 0.001 0.008 4.821 0.002 0.818 0.001 0.001 13.930 0.002 0.048 0.001 0.008 13.760 0.002 0.070 0.001 0.006 6.691 0.002 0.226 0.001 0.001 25.290 0.002 0.048 0.001 0.012 33.560 0.001 0.911 0.001 0.012 16.020 0.002 4.495 0.001 0.009 33.090 0.002 1.107 0.001 0.016 16.300 0.002 0.202 0.001 0.016 28.420 0.002 1.088 0.002 0.012 11.270 0.002 0.071 0.001 0.003 19.770 0.002 0.116 0.001 0.026 8.437 0.002 0.382 0.002 0.011 96.520 0.002 2.469 0.002 0.035 6.913 0.002 0.244 0.001 0.001 21.310 0.002 0.048 0.001 0.017 29.030 0.002 0.076 0.001 0.024 7.938 0.002 0.077 0.001 0.021 1.913 0.002 0.097 0.001 0.013 22.820 0.002 0.088 0.001 0.016 34.800 0.002 0.062 0.001 0.019 28.850 0.002 0.267 0.001 0.013 9.893 0.002 1.142 0.002 0.010 55.700 0.002 23.860 0.001 0.017 468.860 0.002 0.125 0.001 0.013 15.010 0.002 0.066 0.001 0.022 18.000 0.002 0.992 0.001 0.007 18.500 0.002 0.604 0.001 0.023 21.420 0.002 0.475 0.001 0.018 53.410 0.001 0.094 0.002 0.134 31.039 0.002 1.351 0.001 0.001 1.913 0.001 0.048 0.001 5.022 468.860 0.002 23.860 0.002 0.735 67.269 0.000 3.767 0.000 5.474 2.167 0.106 2.789 0.135 Ni Cu Zn As Pb CN 0.002 0.002 0.002 0.003 0.002 0.002 0.001 0.002 0.002 0.002 0.002 0.011 0.001 0.002 0.001 0.002 0.001 0.002 0.001 0.002 0.002 0.001 0.001 0.001 0.002 3.655 0.002 0.002 0.001 0.002 0.002 0.002 0.001 0.002 0.001 0.002 0.001 0.002 0.001 0.002 0.003 0.002 0.002 0.002 0.001 0.001 0.002 0.080 0.001 3.655 0.533 6.694 0.001 0.001 0.001 0.014 0.003 0.014 0.001 0.001 0.002 0.001 0.003 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.002 0.001 0.001 0.001 0.001 0.010 0.001 0.001 0.001 0.001 0.001 0.068 0.001 0.001 0.001 0.010 0.001 0.001 0.001 0.001 0.012 0.001 0.001 0.001 0.001 0.013 0.001 0.004 0.001 0.068 0.010 2.563 0.057 0.046 0.036 0.046 0.032 0.258 0.106 0.321 0.016 0.027 0.040 0.120 0.025 0.036 0.041 0.018 0.001 0.065 0.001 0.022 0.034 0.004 0.022 0.671 0.027 0.027 0.019 0.028 0.021 0.041 0.040 0.089 0.001 0.023 0.105 0.060 0.032 0.019 0.035 0.030 0.564 0.024 0.026 0.020 0.013 0.375 0.028 0.079 0.001 0.671 0.138 1.753 0.002 0.002 0.001 0.002 0.002 0.001 0.005 0.001 0.002 0.002 0.001 0.001 0.002 0.001 0.001 0.001 0.002 0.001 0.001 0.002 0.002 0.002 0.002 0.002 0.002 0.001 0.002 0.002 0.002 0.001 0.001 0.002 0.001 0.001 0.026 0.008 0.002 0.002 0.002 0.001 0.001 0.002 0.001 0.002 0.001 0.002 0.002 0.002 0.001 0.026 0.004 1.567 0.001 0.001 0.001 0.001 0.001 0.003 0.002 0.005 0.001 0.001 0.001 0.001 0.001 0.001 0.010 0.001 0.001 0.001 0.001 0.003 0.001 0.001 0.001 0.073 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.014 0.001 0.001 0.001 0.003 0.001 0.001 0.001 0.001 0.019 0.001 0.001 0.001 0.001 0.002 0.001 0.004 0.001 0.073 0.011 2.896 0.001 0.001 0.001 0.001 0.002 0.001 0.001 0.002 0.001 0.002 0.002 0.001 0.001 0.001 0.002 0.002 0.002 0.002 0.002 0.001 0.002 0.002 0.001 0.002 0.001 0.002 0.002 0.002 0.002 0.002 0.002 0.001 0.002 0.002 0.002 0.002 0.002 0.002 0.002 0.002 0.001 0.002 0.002 0.002 0.002 0.002 0.002 0.002 0.001 0.002 0.000 0.143 4. Results and discussion 4.1. Groundwater level and flow directions Groundwater level surveys provide valuable information on the groundwater gradients and flow directions. This type of survey can also aid qualitative estimates of the flow paths and facilitate a better definition of the extent of the hydrologic system (Figure.2). In this area, there are 2 drainage basin: the right trough (blue arrow) moves down the hill to flat areas. the left trough (red arrow) moves down the hill to flat areas. In the study area can be divided into 4 layers (Figure.3) consisting of weathered or fractured volcanic rock, rhyolite and andesitic tuff (Vw) 53.19%, volcanic rock, rhyolite and andesitic tuff (Vf) 23.40%, floodplain deposit and clay dominant (Qfd) 12.47%. and hard volcanic rock, andesitic and diorite (Vm) 10.64%. Cross section line A Cross section line B Figure.2 Groundwater level and flow directions A B Figure3 Groundwater level, (A)N-S ,(B) E-W - l +C 2- 2+ Ca 2+ g +M EXPLANATION +H CO 3 CO - Cl ANIONS Figure4.Piper diagram 2- SO 4 + 2+ Ca CATIONS 2- 3 - Qfd Vw Vf Vm + +K Na The variables for correlation analysis were T, pH ,EC, TDS,ORP, Salt, SO2-4, PO3−4, NO-3, Cl-,CO32-, HCO-3 ,CN-, Mg2+ , K+, Na+, Ca+, Cr6+ ,Fe, Mn, Ni, As, Al, Co, Cu, Pb and Zn. The correlation matrix for the 27 variables is shown in Table 2. There are significant positive correlations between EC and TDS, EC and salt, TDS and Salt, Mg and SO42-, Aquifer and Cl−, soil and Geology, which indicate that such ions in the groundwater were likely came from the same sources. Mg2+ in groundwater often comes from the input of the dissolution of dolomite in karst areas (Aiuppa et al., 2003; Brenot et al., 2008). Sources of SO4 2− may be rainfall and fertilizers (Edmunds et al., 2003) , sewage effluents and dissolution of sulfide minerals present in granite. In addition, the significantly high positive loading of EC and strong negative loading of pH supporting the hypothesis of water–rock interaction. The EC reflects the amount of minerals dissolved into groundwater, SO 4 4.3. Statistical analysis This study found that these 4 aquifers consist of 6 Groundwater types (Figure.4) in areas and the percentages for each type were Ca-HCO3- (53.19%), CaMg-Cl (14.89%), Ca-Na-HCO3 (8.51%), Ca-Cl (10.64%), Na. -Cl (8.51%), NaHCO3 (4.26%) 2+ Results of chemical analysis and statistics for the 27 physicochemical parameters monitored in 47 groundwater samples shown in Table 1.Average groundwater temperature was 31.88 C. The pH varies from 6 to 8.85 with an average of 7.16. The redox potential was measured within a range of -176.4 – 177.3 mV with an average of 32.02 mV. Also, Table 1 reflected a moderate to high variability (standard deviation and coefficient of variation) among samples of the variables. The highest variability was for Ni followed by Pb, Cu and Al with coefficient of variation values above 1.0, reflecting spatial variation of groundwater quality in area. which positively correlated to TDS and salt. Sodium ion in groundwater derives from the incongruent dissolution of plagioclase in granite, chemical fertilizer, domestic effluents and atmospheric input (Edmunds et al., 2003; Aiuppa et al., 2003; Brenot et al., 2008). Potassium in groundwater often comes from orthoclase and muscovite minerals present in granite, and from pollution sources such as chemical fertilizer. Furthermore, according to correlation result, soil is controlled by weathering rocks in area. Mg 4.2. Results of Chemical characteristics Table 2 The correlation matrix among the chemical constituents for the groundwater samples. T pH ORP EC TDS Salt CO32HCO3K Mn Mg NO3 SO4 PO4 Cl Na Al Ca Cr Fe Co Ni Cu Zn As Cd Pb CN LU Soil Geo Aqifer Topo T pH ORP EC TDS Salt CO3 2- HCO3- 1.000 0.077 0.122 -0.010 0.050 0.001 -0.013 -0.158 0.236 -0.200 0.001 -0.040 -0.094 -0.247 0.224 0.024 -0.088 -0.006 0.167 0.160 -0.039 -0.031 -0.098 0.021 -0.030 0.047 0.028 0.028 0.143 0.080 0.017 0.150 0.073 1.000 0.219 -0.054 0.007 -0.016 0.600 0.097 0.373 -0.093 -0.109 -0.269 -0.216 -0.301 -0.199 -0.206 -0.180 0.219 -0.094 -0.073 -0.142 -0.121 -0.079 -0.036 0.160 -0.054 -0.012 -0.148 -0.070 -0.216 -0.205 -0.167 -0.027 1.000 -0.073 0.007 -0.044 0.014 0.032 -0.046 -0.364 -0.049 -0.122 -0.059 -0.215 -0.234 0.274 0.150 0.044 0.115 0.158 -0.254 0.083 -0.013 0.045 0.289 -0.134 -0.206 0.072 0.024 -0.294 -0.200 -0.191 -0.239 1.000 0.906 0.934 -0.054 0.145 -0.173 0.052 0.700 0.174 0.633 -0.337 -0.191 -0.048 0.086 -0.057 0.223 -0.027 -0.005 0.242 -0.029 -0.041 -0.116 0.081 0.068 0.113 -0.171 -0.205 -0.272 -0.210 0.144 1.000 0.970 -0.085 0.180 -0.112 -0.009 0.719 0.193 0.597 -0.433 -0.230 -0.013 0.072 -0.074 0.187 -0.066 -0.072 0.241 -0.064 -0.108 -0.135 0.084 0.057 0.079 -0.206 -0.233 -0.286 -0.281 0.119 1.000 -0.076 0.177 -0.195 0.022 0.686 0.170 0.580 -0.403 -0.206 -0.080 0.075 -0.074 0.177 -0.054 -0.054 0.239 -0.054 -0.079 -0.137 0.032 0.063 0.092 -0.224 -0.258 -0.330 -0.213 0.104 1.000 -0.074 0.131 -0.048 -0.131 -0.181 -0.109 -0.166 -0.012 -0.157 -0.041 -0.021 -0.079 -0.083 -0.084 -0.038 -0.081 -0.056 0.023 -0.176 -0.064 -0.079 -0.024 -0.201 -0.160 0.066 -0.183 1.000 -0.081 -0.034 0.014 -0.086 -0.097 -0.189 -0.012 -0.074 0.034 -0.080 -0.094 -0.020 0.029 0.049 -0.029 -0.037 0.010 0.281 0.043 0.035 0.018 0.007 0.045 -0.199 -0.251 K Mn Mg NO3 SO4 PO4 Cl Na Al Ca Cr Fe Co Ni Cu Zn As Pb CN LU Soil 1.000 0.011 -0.006 0.012 -0.045 0.044 -0.007 -0.167 -0.145 0.112 -0.195 -0.166 -0.062 -0.015 0.288 -0.088 -0.125 0.061 -0.054 -0.134 0.252 0.092 0.166 0.044 0.192 1.000 0.051 0.160 -0.054 0.086 -0.016 -0.128 -0.105 0.064 -0.056 -0.019 0.103 -0.019 -0.013 -0.068 -0.045 -0.070 -0.021 -0.010 -0.004 -0.148 -0.126 0.032 0.304 1.000 0.173 0.835 -0.120 -0.203 0.087 -0.009 -0.102 0.042 -0.006 -0.012 0.118 -0.058 -0.090 -0.131 -0.013 0.034 -0.112 0.010 -0.064 -0.161 -0.237 0.242 1.000 0.251 0.038 -0.095 0.076 0.069 0.030 0.040 0.075 0.088 0.008 0.058 -0.160 -0.159 -0.086 -0.127 -0.164 0.295 -0.026 -0.004 0.015 0.034 1.000 -0.111 -0.121 0.181 0.114 -0.123 0.034 -0.008 0.106 0.165 0.101 -0.027 -0.138 -0.018 0.056 -0.075 -0.091 -0.027 -0.098 -0.055 0.303 1.000 -0.122 0.060 -0.052 0.029 0.037 0.095 0.113 -0.067 -0.022 0.019 -0.067 -0.005 -0.117 -0.086 0.326 0.584 0.355 -0.002 -0.028 1.000 -0.101 -0.106 -0.065 -0.094 0.230 0.185 -0.089 0.159 0.368 0.034 -0.114 0.590 0.045 0.103 -0.042 -0.035 0.668 0.021 1.000 0.515 -0.052 0.123 0.164 -0.114 -0.048 -0.089 -0.046 -0.034 0.012 -0.053 0.007 -0.043 0.110 0.173 -0.368 -0.142 1.000 -0.016 0.095 -0.044 -0.047 -0.024 -0.039 -0.030 -0.028 -0.092 -0.036 0.110 -0.182 -0.097 -0.064 -0.223 -0.248 1.000 0.095 0.018 0.036 -0.033 -0.067 -0.038 -0.081 0.285 -0.042 0.003 0.075 -0.169 -0.113 -0.045 0.026 1.000 0.497 0.348 -0.009 0.027 0.368 -0.084 0.092 0.117 0.019 -0.114 0.176 0.235 -0.066 -0.084 1.000 0.490 -0.045 0.130 0.622 -0.029 -0.119 0.328 -0.255 0.173 0.307 0.311 0.028 -0.027 1.000 -0.068 0.055 0.498 -0.121 -0.033 0.382 -0.184 0.095 0.337 0.485 0.193 0.228 1.000 0.090 -0.056 -0.047 0.048 -0.036 -0.050 0.059 -0.103 -0.092 -0.064 0.103 1.000 0.182 -0.018 -0.173 0.163 -0.122 0.072 -0.002 -0.008 0.288 0.169 1.000 0.096 -0.131 0.771 -0.005 -0.106 0.115 0.127 0.171 0.065 1.000 0.140 0.017 0.364 0.015 -0.137 -0.114 0.062 -0.106 1.000 -0.052 -0.122 -0.045 -0.015 0.222 0.086 1.000 -0.004 -0.071 -0.044 0.119 -0.016 1.000 0.281 0.248 0.085 0.118 1.000 0.733 1.000 -0.116 -0.221 1.000 0.214 0.118 0.052 1.000 The water quality of old groundwater is another issue taken into account. Generally speaking, the longer the residence time, the higher the concentration of dissolved ions in groundwater. Groundwater tends to evolve chemically toward compositions of sea water along the flow path. It was found by Chebotarev in the Great Artesian Basin that the evolution is normally accompanied by the following regional changes in dominant anion species (Freeze and Cherry 1979) as follows: HCO3 - ⇒ HCO3 -+ SO4-2 ⇒ SO4-2 + HCO3 - ⇒ SO4-2 + Cl ⇒ Cl This is the Chebotarev sequence, expressed in simpler terms as follows: Bicarbonate waters ⇒ Sulfate waters ⇒ Chloride waters The time of travel or residence time determines to a large extent the adoption of local geological conditions. In essence, the chemical composition of groundwater is the result of the prolonged leaching of sedimentary beds. Therefore, the salinity Geo Aqifer Topo concentration of groundwater usually increases with: (1) greater depth, (2) slower drainage, and (3) longer time of exposure. The deeper and/or older the waters, the saltier they are likely to be, and vice versa, in sedimentary basins the chemical composition of groundwater will evolve along its flow-path in an anionic sequence: HCO3- Young groundwater: infiltration, short residence time. HCO3come from carbonate dissolution Low TDS. HCO3 -+ SO4-2 come from dissolution of gypsum and oxidation of pyrite. Then, SO4-2 + Cl- Dissolution of halite that has not been washed away Cl- is old deep groundwater with long residence time and High TDS. Discussion and Conclusion The study results showed that NO3−, Cl−, SO4 2−, PO42- in groundwater derive from sources related to human activities, agricultural fertilizers and sewage effluents. Sodium ion ,Mg2+ and K+ in groundwater derive from the incongruent dissolution of plagioclase, orthoclase and muscovite form the lithologic source. Lead and As in groundwater derive from mineral. The Piper's diagram showed that groundwater changed widely from CaHCO3-Type (53.19%), Ca-Mg-Cl Type (14.89%), Ca-Na-HCO3 Type (8.51%), CaCl Type (10.64%), Na-Cl Type (8.51%) and Na-HCO3 Type (4.26%). In summary both natural and anthropogenic processes were the two major factors for the chemical compositions of groundwater quality around such gold mine area. Reference 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. APPHA, AWWA and WEF. 2005. Standard Methods for the Examination of Water and Wastewater, 21th ed., American Public Health Association, Washington. Aiuppa, A., Bellomoa, S., Bruscab, L., Alessandrob, W.D., Federico, C. 2003. Natural and anthropogenic factors affecting groundwater quality of an active volcano (Mt. Etna, Italy). Applied Geochemistry 18: 863–882 Aravena, R., Auge, M., Bucich, N.1999. Evaluation of the origin of groundwater nitrate in the city La Plata—Argentina, using isotope techniques. Hydrogeology and Land Use Management: 323–327. Brenot, A., Baran, N., Petelet-Giraud, E., Negrel, P., 2008. Interaction between different water bodies in a small catchment in the Paris basin (Brevilles,France): Tracing of multiple Sr sources through Sr isotopes coupled with Mg/Sr and Ca/Sr ratios. Applied Geochemistry 23, 58–75. Compton, J.E., Boone R.D.. 2000. Long-term impacts of agriculture on soil carbon and nitrogen in New England. Forests Ecology : 2314–2330 Chan, H.J., 2001. Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. Journal of Hydrology : 194–210. Conesa, H.M., Garcia G, Faz A, Arnaldos R .2007. Dynamics of metal tolerant plant communities’ development in mine tailings from the ena-La Union Mining District (SE Spain) and their interest for further revegetation purposes. Chemosphere : 1180–1185 Changul,C., Sutthirat, C., Padmanahban, G. , Tongcumpo,C., 2010. Chemical characteristics and acid drainage assessment of mine tailings from Akara Gold mine in Thailand. Environ Earth Sci 60:1583–1595. Edmunds, W.M., Shand, P., Hart, P., Ward, R.S., 2003. The natural (baseline) quality of groundwater: a UK pilot study. Science of the Total Environment 310 (1–3), 25–35. ESRI, 2009. ArcGIS version 9.3. 380 New York Street, Redlands, CA 92373-8100 USA. Farnham, I.M., Johannesson , K.H. , Singh, A.K., Hodged,V.F., Stetzenbach, K.J. 2003. Factor analytical approaches for evaluating groundwater trace element chemistry data. Analytica Chimica Acta 490 : 123– 138 Freeze, R.A. and J.A. Cherry. 1979. Groundwater. Prentice-Hall, Inc. Englewood Cliffs, NJ. 242p. Jiang, Y., Yuan, D., Zhang, C., Zhang, G., He, R., 2008. Impact of land use change on groundwater quality in a typical karst watershed of southwest China. Hydrogeology Journal : 727–735. Jiang, Y., Wue, Y., Groves, C., Yuan, D., Kambesis, P., 2009. Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. Journal of Contaminant Hydrology 109 :49–61. Lin,C.Y., Abdullah,M.H, Praveena,S.M., Yahaya,A.H.B.,Musta,B. 2012.Delineation of temporal variability and governing factors influencing the spatial variability of shallow groundwater chemistry in a tropical sedimentary island Journal of Hydrology:26–42 Ouyang,Y.2005. Evaluation of river water quality onitoringstations by principal component analysis. Water Research 39 :2621–2635. Reimann,C.,Filzmoser,P.,Garrett,R.G.2002. Factor analysis applied to regional geochemical data:problems and possibilities. Applied Geochemistry 17 : 185–206 Acknowledgement : The authors are very grateful to laboratory of the Inter-department of Environmental Science and Graduate School, Chulalongkorn University for partially funding support for financial support.