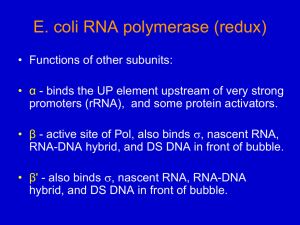
CHAPTER 16
advertisement

CHAPTER 16 Application and Experimental Questions E1. Answer the following questions that pertain to the experiment of Figure 16.7. A. Why was β-ONPG used? Why was no yellow color observed in one of the four tubes? Can you propose alternative methods to measure the level of expression of the lac operon? B. The optical density values were twice as high for the mated strain as for the parent strain. Why was this result obtained? Answer: A. β-ONPG was used to measure the level of expression of the lac operon. More specifically, the cleavage of β-ONPG to produce a yellow compound requires the action of β-galactosidase. Thus the assay is indirectly measuring the amount of β-galactosidase protein. The reason why there was no yellow color in one of the tubes was because the repressor was preventing the expression of the lac operon, and this prevents the expression of β-galactosidase enzyme. Some other methods to measure the expression level of the lac operon could include the following: 1. Conduct a Northern blot to measure the amount of mRNA that is produced from the lac operon. 2. Conduct a Western blot using antibodies against one of the three proteins encoded by the lac operon. 3. Measure the uptake of radiolabeled lactose into the cells. This would measure the amount of the lactose permease that is expressed from the lac operon. B. The merozygote has two copies of the lacZ gene, so it makes twice as much βgalactosidase. E2. Chapter 19 describes a blotting method known as Northern blotting, which can be used to detect RNA that is transcribed from a particular gene or a particular operon. In this method, a specific RNA is detected by using a short segment of cloned DNA as a probe. The DNA probe, which is radioactive, is complementary to the RNA that the researcher wishes to detect. After the radioactive probe DNA binds to the RNA within a blot of a gel, the RNA is visualized as a dark (radioactive) band on an X-ray film. For example, a DNA probe that is complementary to the mRNA of the lac operon could be used to specifically detect the lac operon mRNA on a gel blot. As shown here, the method of Northern blotting can be used to determine the amount of a particular RNA transcribed under different types of growth conditions. In this Northern blot, bacteria containing a normal lac operon were grown under different types of conditions, and then the mRNA was isolated from the cells and subjected to a Northern blot, using a probe that is complementary to the mRNA of the lac operon. [Insert Text Art 16.1] Based on your understanding of the regulation of the lac operon, explain these results. Which is more effective at shutting down the lac operon, the binding of the lac repressor or the removal of CAP? Explain your answer based on the results shown in the Northern blot. Answer: In samples loaded in lanes 1 and 4, we expect the repressor to bind to the operator because no lactose is present. In the sample loaded into lane 4, the CAP protein could still bind cAMP because there is no glucose. However, there really is no difference between lanes 1 and 4, so it does not look like the CAP can activate transcription when the lac repressor is bound. If we compare samples loaded into lanes 2 and 3, the lac repressor would not be bound in either case, and the CAP would not be bound in the sample loaded into lane 3. There is less transcription in lane 3 compared to lane 2, but because there is some transcription seen in lane 3, we can conclude that the removal of the CAP (because cAMP levels are low) is not entirely effective at preventing transcription. Overall, the results indicate that the binding of the lac repressor is more effective at preventing transcription of the lac operon compared to the removal of the CAP. E3. As described in Experimental question E2 and also in Chapter 19, the technique of Northern blotting can be used to detect the transcription of RNA. Draw the results you would expect from a Northern blot if bacteria were grown in media containing lactose (and no glucose) but had the following mutations: Lane 1. Normal strain Lane 2. Strain with a mutation that inactivates the lac repressor Lane 3. Strain with a mutation that prevents allolactose from binding to the lac repressor Lane 4. Strain with a mutation that inactivates CAP. How would your results differ if these bacterial strains were grown in media that did not contain lactose or glucose? Answer: In the normal strain, the lac operon would be fully induced, so there would be maximal expression of the mRNA (lane 1). The same thing would happen in lane 2, because the lac repressor has been inactivated. In lane 3, we would not see any mRNA because the lac repressor does not bind to allolactose. Therefore, the lac repressor would remain bound to the lac operator even in the presence of lactose. In lane 4, the CAP would not activate transcription, although the repressor would not prevent transcription. You would probably see a little bit of transcription, but not as much as the maximal level seen in lane 1. In media without lactose, you would see a band only in lane 2. The only strain that would be induced would be the one that lacked a functional lac repressor. E4. An absentminded researcher follows the protocol described in Figure 16.7 and (at the end of the experiment) does not observe any yellow color in any of the tubes. Yikes! Which of the following mistakes could account for this observation? A. Forgot to sonicate the cells B. Forgot to add lactose to two of the tubes C. Forgot to add β-ONPG to the four tubes Answer: A. Yes, if you do not sonicate, then β-galactosidase will not be released from the cell, and not much yellow color will be observed. (Note: You may observe a little yellow color because some of β-ONPG may be taken into the cell.) B. No, you should still get yellow color in the first two tubes even if you forgot to add lactose because the unmated strain does not have a functional lac repressor. C. Yes, if you forgot to add β-ONPG, you could not get yellow color because the cleavage of β-ONPG by β-galactosidase is what produces the yellow color. E5. Explain how the data shown in Figure 16.9 indicate that two operator sites are necessary for repression of the lac operon. What would the results have been if all three operators were required for the binding of the lac repressor? Answer: The data indicate that two operators are needed because you need O1 plus either O2 or O3 to get a high level of repression. If all three operators were needed, the deletion of any one of them should have prevented high levels of repression. This was not observed. E6. A mutant strain has a defective lac operator site that results in the constitutive expression of the lac operon. Outline an experiment you would carry out to demonstrate that the operator site must be physically adjacent to the genes that it influences. Based on your knowledge of the lac operon, describe the results you would expect. Answer: You could mate a strain that has an F factor carrying a normal lac operon and a normal lacI gene to this mutant strain. Because the mutation is in the operator site, you would still continue to get expression of β-galactosidase, even in the absence of lactose. E7. Let’s suppose you have isolated a mutant strain of E. coli in which the lac operon is constitutively expressed. To understand the nature of this defect, you create a merozygote in which the mutant strain contains an F’ factor with a normal lac operon and a normal lacI gene. You then compare the mutant strain and the merozygote with regard to their β-galactosidase activities in the presence and absence of lactose. You obtain the following results: Amount β-Galactosidase Addition of (percentage of mutant strain Lactose in the presence of lactose) Mutant No 100 Mutant Yes 100 Merozygote No 100 Merozygote Yes 200 Explain the nature of the defect in the mutant strain. Answer: It appears to be a mutation in the operator site, so that a repressor protein cannot bind there and prevent lac operon expression in the absence of lactose.