Energy Systems & Climate Change – week 4 Winter – Zita solns
advertisement

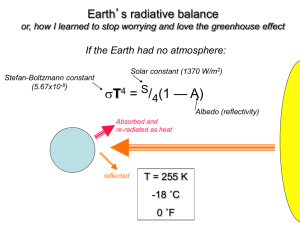
Energy Systems & Climate Change – week 6 Winter – Zita solns – Friday 17 Feb. 2012 Wolfson Ch.13: Forcing the Climate Q 1, 4, 5, 6 / AYC 1, 2 / Ex 4, 6, 7, 9, 10 Questions 1, 4, 5, 6: 1. Positive feedback is destabilizing. Positive feedback cycles return on themselves, and amplify. Some positive warming feedbacks: * Water vapor feedback: climate warms → water evaporates → atmospheric H20 is a greenhouse gas → climate warms more … * Ice-albedo feedback: ice melts lower albedo less sunlight is reflected more heat is absorbed more ice melts … * Earth warms → more water evaporation → more clouds → outgoing infrared is absorbed → clouds re-radiated infrared → Earth warms some more .. * Permafrost melt / methane release: warming permafrost melting methane released more heating more melting more methane released … * Climate warms people use more energy for air conditioners power plants emit more GHG climate warms more … A positive cooling feedback: * Earth cools rapidly (e.g. by a Milankovitch mechanism or volcanoes) snow & ice advance higher albedo less solar radiation absorbed more cooling more snow & ice … snowball Earth Negative feedback is stabilizing. Negative feedback cycles return to the opposite effect, and damp. * Earth warms slowly radiates more energy Earth cools back toward equilibrium temperature Energy\1112\WINTER\HW\5W13soln.doc -1- * Earth warms slowly → CO2 and water vapor in atmosphere increase → greater precipitation causes more weathering of rock, removing CO2 from atmosphere → Earth cools * Earth warms → more evaporation → more clouds → higher albedo → less sunlight absorbed → Earth cools * atmospheric CO2 increases → plants thrive → plants remove some CO2 from atmosphere * Earth warms → soils dry → plants die → land albedo increases → less sunlight absorbed → Earth cools 4. The microphone example is a positive (destabilizing) feedback. Signal input into the microphone gets over-amplified when you stand in certain positions with respect to the speakers (due to the capacitance of your body), and the sound comes out too loud and distorted. 5. CO2 molecules don’t just go into the atmosphere. They also are absorbed by plants, which are eaten by animals, and CO2 percolates into and out of the ocean. So the lifecycles and decay times of plants and animals, and ocean circulation times, also contribute to absorption and release times of CO2, which can vary from decades to thousands of years. 6. The whole Earth radiates energy away (Asphere = 4πR2). Only ¼ of the Earth effectively absorbs sunlight (Adisk = π R2). Therefore the net solar insolation is reduced by this geometrical factor of 4. Exercises 4, 6, 7, 9, 10: 4. Using a climate sensitivity of 0.5 K per Watt per square meter (in the middle of the range given in the text), estimate how much of the 0.9C global temperature increase since 1900 could be due to solar forcing, whose value in Figure 13.4 is in the range of 0.06 to 0.24 W/m2. dT Solution: dT F dF dT K dTmin Fmin 0.5 0.06 W 2 0.03K 3% of the warming m W 2 dF m dT K dTmax Fmax 0.5 0.24 W 2 0.12 K 13% of the warming m W 2 dF m (Note that greenhouse gases are currently responsible for about 20 times more warming than the Sun, whose luminosity fluctuations are only about 0.1 at solar max.) 6. Exercise 5 shows that a doubling of preindustrial CO2 amounts to a forcing of about 3.7 W/m2. Given a best-guess climate sensitivity of 3C for a doubling of CO2, find the corresponding climate sensitivity when expressed in Kelvins per Watt per square meter. Energy\1112\WINTER\HW\5W13soln.doc -2- dT double CO2 dT 3K K Solution: 0.81 W 2 dF dF 3.7 W 2 double CO2 m m 7. The density of gasoline is about 6 pounds per gallon, and carbon accounts for nearly all the weight of gasoline. Show that combustion of 1 gallon gasoline produces about 20 pounds of CO2 (the exact value is closer to 22 pounds). Solution: Combustion of carbon: C O2 CO2 Molecular weights: 12 2 16 12 32 44 weight of CO2 44 CO2 produced = gasoline burned 6 lb 22 lb CO 2 12 weight of C 9. Figure 13.10 shows global fossil carbon emissions of about 9 Gt per year. Given that the United States accounts for about one-fourth of global emissions, estimate the U.S. annual per capita emissions of CO2 (not carbon; see Box 5.1). Solution: Annual carbon emissions are ¼ 9 Gt 9/4 Gt of carbon. The atomic mass of C is 12, and that of O is 16, so CO2 is 12 16 16 44. To convert carbon mass to CO2, use the mass ratio: 44 CO 2 9 Gt Carbon 8.25Gt CO 2 . 4 12 C There are about 310 million people in the United States, so this is about 8.25 109 tons CO2 per year 27 tons of CO2 per person per year 310 106 people (We emitted “only” 20 tons per person, just a few years ago when Ed.1 of this text was written. Uh-oh.) 10. Figure 13.10 shows that we humans have added about 240Gt of carbon to the atmosphere during the industrial era. If the dominant removal mechanism is the transfer of carbon to the deep ocean, use the flows shown in Figure 13.10 to obtain a crude estimate of the time it would take to remove all this anthropogenic CO2, and compare your answer with the 300- to 1,000-year CO2 lifetime discussed in Section 13.5. Solution: The mass of carbon to be removed is M0 240 Gt. From Figure 13.10, removal to the deep ocean is determined by the difference between the biological pump (11 Gt/yr down) and the upwelling/diffusion (9 Gt/yr up), plus a bit of sedimentation (0.2 Gt/yr.) So the net flow down to the deep ocean is dM/dt 2.2 Gt/year. Energy\1112\WINTER\HW\5W13soln.doc -3- If the sequestration process is linear, then the mass of carbon in the atmosphere at some later time t is M(t) = M 0 t dM dt (assuming no more carbon is put into the atmosphere!) How long would it take until M(t) 0, that is, until all the atmospheric carbon is removed to the deep ocean? Solve for t: dM 0 M0 t dt dM M0 t dt M0 240 Gt t 109 years dM 2.2 Gt/yr dt This is an unrealistically optimistic estimate. 300 to 1000 years is more likely, since the sequestration process is not truly linear; the more acidic the ocean grows, the slower its sequestration rate becomes; and we are still pumping CO2 into the atmosphere, therefore into the ocean. Carbon would remain in the atmosphere after 1000 years even if emissions ceased. Argue Your Case: 1. The natural flows have been in equilibrium. The anthropogenic input, while smaller, significantly disrupts the natural equilibrium. 2. The steep rise in atmospheric CO2 correlates with the onset and rapid increase of fossil fuel burning since the industrial age. What’s more, radioisotope analysis proves that this influx is depleted in C14. It can only have come from ancient, that is, fossil carbon, not from burning fuel grown within the last few thousand years. 3. Sulfates cause respiratory problems and reduce sunlight needed for crops. They dissipate from the atmosphere on short timescales unless injection is repeated and continued. Their cooling potential is weak and their side effects are severe. And they do not remove the atmospheric CO2 which is acidifying the oceans. Energy\1112\WINTER\HW\5W13soln.doc -4-