Interactions of Dissolved Organic Matter with Mercury in the Florida
advertisement

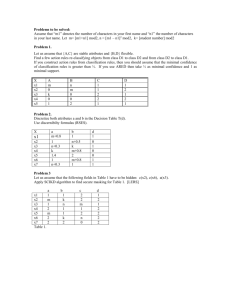
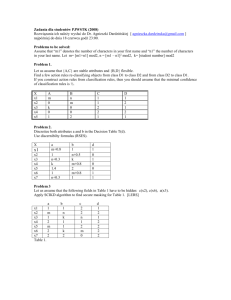
Interactions of Dissolved Organic Matter with Mercury in the Florida Everglades George Aiken U.S. Geological Survey, Boulder, CO Interactions of mercury (Hg) with dissolved organic matter (DOM) are hypothesized to play important roles in controlling the reactivity, bioavailability and transport of Hg in the Everglades. Little is known, however, about how Hg interacts with DOM or how strong these interactions are. The questions being addressed by this research are: 1) By what mechanisms and how strongly does Hg interact with DOM, and 2) What role do these mechanisms play in controlling the effects that Hg has on organisms. This research is driven by the hypothesis that the chemistry and structural characteristics of the DOM in the Everglades strongly influence the processes that control Hg cycling and bioavailability in the environment. A combined field/laboratory approach designed to characterize DOM in the Everglades and to relate the structural characteristics of the DOM to its reactivity with Hg is being used to test this hypothesis. Most of the DOM in the Everglades originates from the degradation and leaching of organic detritus derived from the algae, bacteria and macrophytes living within the wetland environment. In addition, organic matter is also transported to the Water Conservation Areas of the Everglades in the canals that drain the Everglades Agricultural Area. Areas strongly influenced by the Everglades Agricultural Area had higher dissolved organic carbon (DOC) concentrations, had a greater amount of hydrophobic acids and hydrophobic neutrals, and the DOM was more aromatic than samples collected from less impacted areas. Pore waters were found to contain greater DOC concentrations than overlying surface waters, with the highest DOC concentrations in pore waters from the more eutrophic study areas. DOC concentrations and the contributions of aromatic organic molecules to the pool of molecules comprising the DOM were lower in those areas with higher concentrations of methylmercury. The amount and nature of DOM in the Everglades was found to be dependent on the dominant vegetation types, hydroperiod, interactions of surface water with peat pore waters, and interactions with canal water. To better define the nature and strength of interactions between DOM and Hg, the hydrophobic acid fraction (HPOA), hydrophilic acid fraction (HPIA), hydrophobic neutral fraction (HPON), fulvic acid and humic acid were isolated from various surface waters in the Florida Everglades. Using these isolates, Hg-DOM binding constants were determined over a wide range of Hg(II) to DOM concentration ratios by means of a modified equilibrium dialysis ligand exchange method. Very strong interactions (KDOM' = 1023.20.5 L kg-1 at pH = 7.0 and I = 0.1), indicative of Hg-thiol bonds, were observed at Hg(II)/DOM ratios below approximately 1 g Hg(II) per mg DOM. Hg(II)/DOM ratios above approximately 10 g Hg(II) per mg DOM gave much lower KDOM' values (1010.70.5 L kg-1 at pH = 4.9 to 5.6 and I = 0.1), consistent with Hg(II) binding mainly to oxygen functional groups. These results suggest that the binding of Hg(II) to DOM under natural conditions (very low Hg(II)/DOM ratios ranging from 0.01 to 10 ng of Hg/mg of DOM) is controlled by a small fraction of DOM molecules containing reactive thiol functional groups. Similar strong Hg-DOM binding constants were obtained in studies of the partitioning of Hg(II) between DOM and particulate organic matter (POM). In this work, the HPOA isolate from F1, a northern, eutrophic site, was more effective at competing with POM for Hg(II) than the sample from 2BS, a more pristine site. Under most environmental conditions, therefore, it can be expected that only the strongest DOM sites will interact with Hg(II). In the case of fully oxygenated Everglades water (sulfide-free), the binding of Hg(II) by DOM should dominate Everglades’ dissolved inorganic mercury speciation. However, even for the case of strong binding sites (KDOM' = 1023.20.5), Hg-sulfide complexes are predicted to dominate dissolved inorganic Hg solution speciation in the presence of small concentrations (nanomolar) of sulfide because of the strong sulfide affinity for Hg. Where measurable total sulfide concentrations are present in the surface water and pore water of the Everglades, therefore, Hg-sulfide complexes likely predominate. A chemical equilibrium approach does not completely explain the behavior of Hg(II) in the presence of DOM, however. For instance, chemical speciation models indicate that pore waters in the Everglades, especially in the eutrophic areas, are supersaturated with respect to cinnabar (mercuric sulfide, HgS); however, no cinnabar has been found in the peat soils of the Everglades. Therefore, to better define the geochemical interactions between DOC, Hg(II) and sulfide, experiments were designed using the organic matter isolates and whole water samples to study interactions of DOC with Hg in cinnabar dissolution and precipitation experiments. Cinnabar is a relatively insoluble solid (log Ksp = -52.4) under most environmental conditions, but, in the presence of DOM, particularly the humic fractions (HPOA, humic acid, and fulvic acid), a significant amount of Hg (up to 1.7 M/mg C) was released from cinnabar over a period of seven days at pH 6.0. The amount of Hg dissolved by various fractions of organic matter followed the order: humic acid > HPOA fulvic acid >> HPIA. The hydrophobic and hydrophilic neutral fractions dissolved insignificant quantities of Hg from cinnabar. Model compounds such as cysteine and thioglycolic acid dissolved small amounts of Hg from the cinnabar surface, while other model compounds such as acetate, citrate, and EDTA dissolved no detectable Hg. There was a positive correlation (R2 = 0.84) between the amount of Hg released and the aromatic carbon content of the DOM. Precipitation and aggregation of metacinnabar (black HgS) was inhibited in the presence of low concentrations of humic fractions of DOM isolated from the Florida Everglades. At low Hg concentrations (5x10-8 molar (M)), DOM prevented the precipitation of metacinnabar. At moderate Hg concentrations (5x10-5 M), DOM inhibited the aggregation of colloidal metacinnabar (Hg passed through a 0.1 m filter, but was removed by centrifugation). At Hg concentrations greater than 5x10-4 M, mercury formed solid metacinnabar particles that were removed from solution by a 0.1 m filter. Organic matter rich in aromatic moieties was preferentially removed with the solid. HPOA, humic and fulvic acids inhibited aggregation better than HPIA. Inhibition of metacinnabar precipitation appears to be the result of strong DOM-Hg binding. Prevention of aggregation of colloidal particles appears to be caused by adsorption of DOM and electrostatic repulsion. A general observation that can be drawn from all of our studies of Hg(II)-DOM interactions is that the amount of dissolved Hg(II) present in a given system is greater in the presence of reactive DOM. This is significant because many of the processes (both abiotic and biotic) involved in Hg cycling in the Everglades are hypothesized to be strongly dependent on the concentration of total dissolved Hg. Preliminary results of ongoing wetland enclosure (mesocosm) experiments in the Everglades are consistent with this hypothesis. In these experiments, mesocosms amended with the most reactive organic matter from the Everglades, the HPOA fraction of the DOM obtained from site F1, contained higher concentrations of total dissolved Hg than control enclosures containing less reactive, native DOM. The DOM amended enclosures also were found to have enhanced methylation (both biotic and abiotic pathways) of Hg(II) and enhanced photo-oxidation of methylmercury relative to the controls. Future research will address the mechanisms by which DOM influences these important reactions. Aiken, George, U.S. Geological Survey, 3215 Marine Street, Boulder, CO, 80303 Phone: 303-541-3036, FAX: 303-447-2505, graiken@usgs.gov