ventilation_class_notes - Carteret Community College
advertisement

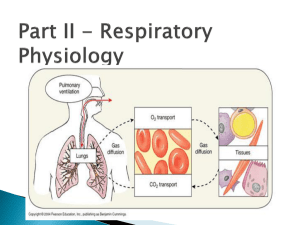
Ventilation Ventilation is the process that exchanges gases between the external environment and the alveoli of the lungs. Through ventilation, oxygen is carried to the alveoli from the environment, and carbon dioxide is carried away from the alveoli. Other, inert gases also move in and out of the lungs with oxygen and carbon dioxide. Understanding ventilation is essential for respiratory care practitioners, who need to understand the following three key areas. How the excursion of the diaphragm changes the intra-alveolar and intrapleural pressures Lung characteristics (static and dynamic) Normal and abnormal ventilatory patterns PRESSURE DIFFERENCES ACROSS THE LUNGS Key Facts: Gas will always travel from an area of higher pressure to an area of lower pressure All pressures are expressed in mm Hg In order to understand the pressure characteristics of the lungs, you need to know the following four definitions and formulas: Driving pressure o The pressure difference between two points in a tube or vessel, and thus the force moving gas or fluid through a tube or vessel o Calculation: Driving Pressure = (P1-P2) o Example: If P1 = 20 mmHg, and P2 = 5 mmHg, then 20 mmHg - 5 mmHg = 15 mmHg Transairway pressure o The difference between the mouth pressure and the alveolar pressure. o Calculation: Pta = (Pm - Palv) o Examples: Inspiration: 760 - 757 = 3 mmHg Gas moves into the lungs Expiration: 760 - 763 = -3 mmHg Gas moves out of the lungs Transpulmonary pressure o The difference between alveolar pressure and pleural pressure. o Calculation: Ptp = (Palv - Ppl) o Examples: Inspiration: 760 - 755 = 5 mmHg Expiration: 763 - 758 = 5 mmHg The Ppl is always subatmospheric but is less negative during expiration as compared with inspiration (758 vs. 755) Transthoracic pressure o The difference between the alveolar pressure and the body surface pressure. o Calculation: Ptt = (Palv - Pbs) o Examples: Inspiration: 757 - 760 = -3 mmHg Gas moves into the lungs Expiration: 763 - 760 = 3 mmHg Gas moves out of the lungs THE ROLE OF THE DIAPHRAGM IN VENTILATION The flow of gas in and out of the lungs is caused by airway pressure changes, which are created by the actions of the diaphragm. During inspiration, the diaphragm contracts and moves downward. Thoracic volume increases, and intrapleural and intra-alveolar pressures decrease. Intra-alveolar pressure is less than barometric pressure, allowing gas to move from the atmosphere down the tracheobronchial tree across this pressure gradient until the two pressures are in equilibrium. This point is known as end-inspiration. During expiration, the diaphragm relaxes and moves upward. Thoracic volume decreases, and intrapleural and intra-alveolar pressures increase. Intra-alveolar pressure is greater than the barometric pressure, allowing gas to move out of the lungs across this pressure gradient until the intraalveolar pressure and the barometric pressure are, once again, in equilibrium, known as end-expiration. During normal inspiration and expiration, the intrapleural pressure is always less than the barometric pressure. The following graphic shows the effect of the diaphragm on lung pressures and gas flow. At rest, the normal excursion of the diaphragm is about 1.5 cm and the normal intrapleural pressure change is about 2-4 mmHg (3-6 cmH2O) o mmHg x 1.5 = cmH2O Deep inspiration o the diaphragm may move 6-10 cm (2-4 in), which can cause the average Ppl to drop as low as -50 cm H2O below Pb Forced expiration o the Ppl may raise 70-100 cmH2O above Pb POSITIVE PRESSURE VENTILATION Positive pressure ventilation significantly affects the normal interaction between the diaphragm and lung pressures. When a patient receives positive pressure ventilation, during inhalation, the patient’s intra-alveolar pressure rises above atmospheric pressure as gas is pushed into the lung by the machine. As positive pressure increases in the alveoli during inspiration, the intrapleural pressure also increases, gradually reaching about 30 cm H20 above its normal resting level (which is normally below atmospheric pressure). During exhalation, the intra-alveolar pressure decreases toward atmospheric pressure. The intrapleural pressure also decreases to its resting level (below atmospheric pressure). At end-expiration, the intra-alveolar pressure is in equilibrium with atmospheric pressure, and intrapleural pressure is held at its resting level. STATIC CHARACTERISTICS OF THE LUNGS Static refers to the study of matter at rest and the forces resulting in or maintaining equilibrium. The lungs have a natural tendency to recoil inward - collapse The chest wall has a natural tendency to move outward - expand The lungs are at their resting volume when the inward recoil force of the lungs is equal to the outward force of the chest wall FRC: the volume remaining in the lungs when the recoil pressure of the lungs and the outward pressure of the chest wall equilibrate during normal quiet breathing. There are two major forces in the lungs that cause an inflated lung to recoil inward: 1. the elastic properties of the lungs 2. the surface tension produced by the layer of fluid that lines the alveoli Elastic Properties of the Lungs How readily the elastic force of the lungs accepts a volume of inspired air is known as lung compliance o the ease in which the lung distends and fills Compliance determines how many units of air (liters or milliliters) the lungs can accommodate for each centimeter of transpulmonary pressure change (cm H2O). Example: if a person generates a negative intrapleural pressure of 5 cm H2O during inspiration and the lungs accept a new volume of 0.5 L of gas, the CL of the lungs would be expressed as: CL = V (L) = 0.5 L = 0.1 L/cm H2O P (cmH2O) 5 cm H2O The change in driving pressure can be either: o negative (during spontaneous breathing) drop in Palv to -5 cmH2O o positive (during mechanical ventilation) increase in Pm to 5 cmH2O o compliance is always expressed as a positive number At rest, the average lung compliance for each lung is about 0.1 L/cm H 2O. o 100 mL of air is delivered into the lungs per 1 cm H2O pressure change o When lung compliance increases, the lungs accept a greater volume of gas per unit of pressure change (i.e. 120 mL). o When lung compliance decreases, as occurs with many lung diseases, the lungs accept a smaller volume of gas per unit of pressure change (i.e. 80 mL) CL also decreases as the alveoli approach their filling capacity. o The elastic force of the alveoli steadily increases as the lungs expand which lowers the ability of the lungs to accept more gas. This volume/pressure relationship is shown in the following graphic. Hooke’s Law Hooke’s Law helps explain compliance by describing elastance, the natural ability of matter to respond to force and to return to its original shape after force is no longer exerted. Elastance is the opposite of compliance. Lungs with high compliance have low elastance, and lungs with low compliance have high elastance. o Formula: ∆P/∆V Hooke’s Law states that elastic bodies respond to force in a predictable and measurable way. o When an elastic body is acted on by 1 unit of force, the elastic body will stretch one unit of length. o When acted on by 2 units of force, it will stretch 2 units of length, etc. o When the force goes beyond the elastic limit of the substance, the ability of the length to increase stops. o If the force continues to rise, the elastic substance will break. When applied to the lungs, volume is substituted for length, and pressure is substituted for force. o Volume varies directly with pressure until the elastic limit of the lung unit is reached. At this point little or no volume occurs. If the pressure continues to rise, the lung unit will rupture. o Hooke’s Law helps explain why hazards such as pneumothorax (resulting from the rupture of alveolar sacs) can occur with the increased pressure of mechanical ventilation. SURFACE TENSION AND ITS EFFECT ON LUNG EXPANSION In order to understand how the liquid that lines the inner surface of the alveoli can affect lung expansion, an understanding of the following is essential: 1. surface tension 2. Laplace’s Law 3. the role of pulmonary surfactant Surface Tension Surface tension is the attraction between gas and liquid molecules. Surface tension is measured in dynes per centimeter o One dyne/cm is the force necessary to cause a tear 1 cm long in the surface layer of a liquid the liquid inside the interior alveolar surface can exert more than 70 dynes/cm this can easily cause complete alveolar collapse Surface tension is a property of the fluid and is constant for any specific fluid Laplace’s Law Describes how the distending pressure of a liquid bubble is influenced by: 1. the surface tension of the bubble 2. the size of the bubble itself If there is one liquid - gas interface (bubble in liquid), then P = 2ST r P = pressure difference ST = surface tension r = radius of the liquid sphere (cm) 2 = factor If there is two liquid - gas interfaces (soap bubble on end of a tube), then P = 4ST r P = pressure difference ST = surface tension r = radius of the liquid sphere (cm) 4 = factor Laplace’s Law shows that the distending pressure of a liquid sphere is: 1. directly proportional to the surface tension of the liquid a. As the surface tension of a liquid bubble increases, the distending pressure needed to hold the bubble open increases 2. inversely proportional to the radius of the sphere a. When the radius of a liquid bubble increases, the pressure necessary to keep the bubble open decreases When 2 different size bubbles, having the same surface tension, are in direct communication, the greater pressure in the smaller bubble will cause it to empty into the larger bubble. Surface tension is the same in both the small and large bubble; it's the distending pressures that change. Laplace's Law Applied to the Alveolar Fluid Lining On the inner surface of the alveoli is fluid that can resist lung expansion. Since the liquid film that lines the alveoli resembles a bubble, a high transpulmonary pressure must be generated to keep the small alveoli open. This collapsing tendency is offset by pulmonary surfactant which significantly lowers surface tension How Pulmonary Surfactant Regulates Alveolar Surface Tension Pulmonary surfactant is a complex substance made of phospholipids (90%) and proteins (10%) that is produced by the alveolar type II cells. o Pulmonary surfactant works to lower surface tension at the alveolar gas-liquid interface. The primary chemical is DPPC (dipalmitoyl phosphatidylcholine), a unique molecule with water-soluble and water-insoluble ends that allow it to bridge the interface and promote gas-liquid interchange by lowering the surface tension there. The DPPC molecule at the alveolar gas-liquid interface causes surface tension to decrease in proportion to its ratio to alveolar surface area o when the alveolus decreases in size (expiration), the proportion of surfactant to alveolar surface area increases, increasing the physiologic effect of the pulmonary surfactant o when the alveolus increases in size (inspiration), the amount of surfactant to alveolar surface area decreases the number of surfactant molecules doesn't change when the size of the alveolus changes this is not significant because the distending pressure decreases as the radius of the alveolus increases In the absence of pulmonary surfactant, the alveolar lining fluid behaves according to Laplace’s law: o High intrapleural pressure is needed to keep the alveoli open. o Lack of sufficient offsetting pressure can result in the collapse of the alveoli, which is called atelectasis. o In order to keep the alveoli open in situations of low surfactant, the patient experiences a significant increase in the work of breathing. Causes of Pulmonary Surfactant Deficiency GENERAL CAUSES Acidosis Hypoxia Hyperoxia Atelectasis Pulmonary vascular congestion SPECIFIC CAUSES Adult respiratory distress syndrome (ARDS) Infant respiratory distress syndrome (IRDS) Pulmonary edema Pulmonary embolism Pneumonia Excessive pulmonary lavage or hydration Drowning Extracorporeal oxygenation (ECMO) DYNAMIC CHARACTERISTICS OF THE LUNGS In terms of the lungs, dynamic refers to the movement of gas in and out of the lungs, as well as the pressure changes needed to move the gas. To understand the lungs’ dynamic characteristics, you need to understand Poiseuille’s law for flow and pressure, as well as the airway resistance equation. Poiseuille’s Law Arranged for Flow During normal inspiration and expiration, the bronchial airways lengthen and shorten, respectively. These changes really don’t make much difference during normal breathing, but with certain respiratory disorders, such as asthma and emphysema, flow and pressure can be impacted significantly. The equation: V= ∆Pr4π 8l n Poiseuille’s equation states that flow is directly proportional to pressure (P) and the radius (r4) of the vessel or tube. Flow is also inversely proportional to tube length (l) and viscosity (n) of the gas or fluid. Thus, flow will decrease when pressure and tube radius decrease. Flow will also decrease in response to increased tube length and increased viscosity. As anyone with asthma knows, flow is profoundly affected (shown as a power of 4 in the equation) by the radius of the tube or vessel. For example, if pressure remains constant, reducing the radius of a tube by half reduces the gas flow to 1/16 of the original flow. Similarly, decreasing a tube radius by 16 percent decreases the gas flow to onehalf of the original rate. Poiseuille’s Law Arranged for Pressure The equation: P= V8l n r4π Poiseuille’s law arranged for pressure states that pressure is directly proportional to flow, tube length, and viscosity, and it is inversely proportional to tube radius. Thus, pressure decreases in response to decreased flow rate, tube length, and viscosity, while it increases in response to a decreased tube radius. Pressure is also profoundly affected by the tube radius. If the radius of a bronchial tube with a driving pressure of 1 cm H2O is reduced in size by half due to swelling, then the driving pressure through the bronchial tube has to increase 16 times (16 cm H2O) to maintain the same flow rate. Similarly, decreasing a tube radius by 16 percent requires a pressure of twice its original level to maintain the same flow. Poiseuille’s Law Rearranged to Simple Proportionalities Poiseuille’s law rearranged to simple proportionalities simply states that flow diminishes during exhalation because the bronchial airway radius decreases. However, the actual change in flow in normal exhalation is minimal. If gas flow is to remain constant during exhalation, the driving pressure must increase to maintain a constant gas flow. Again, during normal exhalation, this is not a factor, but in people with respiratory disorders such as emphysema, the flow reductions and transthoracic pressure increases may be significant. AIRWAY RESISTANCE Airway resistance is the pressure difference between the mouth and the alveoli (transairway pressure), divided by the flow rate. Airway resistance is measured in centimeters of water per liter per second. Normal airway resistance in adults is about 0.5 to 1.5 cm H2O/L/sec. Patients with chronic obstructive pulmonary disease (COPD) and infants have much higher airway resistance. Air moves through the bronchial airways in two ways: Laminar flow is a streamlined gas flow o the gas molecules move through the tubes in a pattern parallel to the sides of the bronchial tubes o It occurs at low flow rates and low pressure gradients. Turbulent flow is a random gas flow o The gas encounters resistance from the tubes and from other molecules. o It occurs at high flow rates and high pressure gradients. TIME CONSTANTS Time constant is product of airway resistance and lung compliance. It is the time (in seconds) needed to inflate a lung region to 60 percent of its capacity. If all variables are constant and the airway resistance in a lung region doubles, time constant will double as well. If lung compliance is reduced by half, time constant will also be halved, as will the lung region’s capacity to fill. DYNAMIC COMPLIANCE How readily a lung fills with gas during a specific time period is called dynamic compliance. Dynamic compliance differs from static compliance in that dynamic compliance is determined during a period of actual gas flow; static compliance is determined during a period of no gas flow. In healthy lungs, the dynamic compliance is roughly equal to static compliance at all breathing frequencies. VENTILATORY PATTERNS The Normal Ventilatory Pattern The ventilatory pattern consists of: 1. Tidal volume a. the volume of air that normally moves into and out of the lungs in one quiet breath b. Normal value: 7-9 mL/kg (3-4mL/lb) of ideal body weight c. Ideal Body Weight (lbs) i. Male: 106+6(H-60) ii. Female: 105+5(H-60) 1. H=height in inches 2. Ventilatory rate a. the number of breaths per minute b. Normal value: 10-20 bpm 3. Relationship between inhalation and exhalation (I:E Ratio) a. Normally considered 1:2 b. The actual gas flow is 1:1 but “2” includes pause at end expiration c. Three equal phases: i. inspiratory phase ii. expiratory phase iii. pause at end-expiration Alveolar Ventilation versus Dead Space Ventilation Alveolar ventilation is the portion of the tidal volume that reaches the alveoli and participates in gas exchange Any gas that does not reach the alveoli is not effective and is called dead space ventilation. Dead space is ventilation without perfusion. Three types of dead space: o Anatomic dead space the volume of gas in the conducting airways: nose, mouth, pharynx, larynx, lower airways, and terminal bronchioles Normally 1 mL / lb (2.2 mL / kg) 1kg=2.2 lbs because of anatomic dead space, the gas that enters the alveoli during inspiration is actually a combination of dead space gas and fresh gas o Alveolar dead space Occurs when an alveolus is ventilated but not perfused with pulmonary blood the air that enters the alveolus is not effective in terms of gas exchange. This can occur when a blood clot, called a pulmonary embolus, blocks pulmonary blood flow to a portion of the lung. o Physiologic dead space The sum of the anatomic dead space and alveolar dead space. Normally, 0.2 – 0.4 Calculation for Alveolar Ventilation Formula: VA = (VT - VD) x f Example: If VT = 500 mL, VD = 150 mL and f = 12, then: (500-150) x12 = 4200 mL = 4.2L The Effect of Breathing Depth and Frequency on Alveolar Ventilation Subject A B C VT (mL) 150 500 1000 Frequency (f) 40 12 6 VE (mL) 6000 6000 6000 VD (mL) 150* x 40 = 6000 150 x 12 = 1800 150 x 6 = 900 VA (mL) 0 4200 5100 *Individual’s ideal body weight is 150 lbs Significance: An increased depth of breathing (tidal volume) is far more effective than an increased rate in increasing total alveolar ventilation. HOW NORMAL INTRAPLEURAL PRESSURE DIFFERENCES CAUSE REGIONAL DIFFERENCES IN NORMAL LUNG VENTILATION Regional differences in lung ventilation are caused by: In the upright lung: 1. Intrapleural pressure is always below atmospheric pressure (Pb) during both inspiration and expiration 2. Intrapleural pressure gradients exist from the upper lung region to the lower lung region 3. The negative intrapleural pressure at the apex is normally greater (-7 to 10 cmH2O) than at the base (-2 to -3 cmH2O) Average: -3 to -6 cmH2O 4. Changes due to: a. gravity b. distribution of weight in the lungs c. lungs are suspended at the hilum d. lung base weighs more than the apex (increased blood flow) 5. Greater negative pressure in the upper regions causes the alveoli in those areas to be more expanded than alveoli in the lower regions. a. Many alveoli are close to or at their total filling capacity. b. The compliance of alveoli in the upper regions is lower than the compliance of alveoli in the lower regions. c. Ventilation is much greater and more effective in the lower lung regions. THE EFFECT OF AIRWAY RESISTANCE AND LUNG COMPLINACE ON VENTILATORY PATTERNS Ventilatory patterns can change due to changes in lung compliance and airway resistance. For example, a person may adopt a ventilation pattern based on expenditure of energy rather than on efficiency of ventilation. Condition Decreased CL Increased Raw Frequency (f) Increased Decreased Tidal Volume (VT) Decreased Increased These patterns decrease the work of breathing for the individual The pattern adopted may not be most efficient in terms of physiologic gas exchange OVERVIEW OF SPECIFIC VENTILATORY PATTERNS The following are the most frequently seen ventilatory patterns. Apnea: absence of spontaneous ventilation. Without intervention, death follows in minutes. Eupnea: normal, spontaneous breathing Biot’s respiration: short episodes of rapid, uniformly deep inspirations, followed by 10 to 30 second periods of apnea; first noted in patients with meningitis Hyperpnea: increased volume of breathing with or without increased frequency Hyperventilation: increased alveolar ventilation due to increased breathing depth or rate; a breathing pattern resulting in an arterial carbon dioxide level below normal. Note that a rapid rate may or may not result in hyperventilation, depending on the underlying lung disease. Hypopnea: decreased rate and depth of breathing Hypoventilation: decreased alveolar ventilation due to decreased breathing depth or rate; a breathing pattern resulting in an arterial carbon dioxide level above normal Tachypnea: rapid breathing rate Cheyne-Stokes respiration: 10 to 30 seconds of apnea, followed by a gradual increase in breathing volume and frequency, which is then followed by a gradual decrease in breathing volume and frequency; associated with cerebral disorders Kussmaul’s respiration: increased breathing depth and rate; commonly associated with diabetic ketoacidosis Orthopnea: when a person is able to breathe most comfortably in the upright position only Dyspnea: difficulty in breathing that the person is aware of. Note that unconscious people cannot have dyspnea.