Contamination In the Ozark Aquifer
advertisement

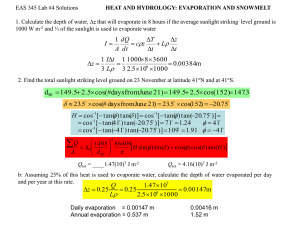
Contamination in the Ozark Aquifer Created By: John Goldsmith, 27 April 2010 Emporia State University GO571 Hydrogeology Aquifer Background Information The Ozark aquifer is a large significant source of water for communities, rural districts, industry, and much agriculture in Southern Missouri, Southeast Kansas, and Northern Oklahoma. There are several areas of the aquifer; each area has its own name and individual characteristics. The area extending into southeast Kansas from southwest Missouri is the confining system of the aquifer. The area of most interest with contamination is in the Tri-State mining area in the region where Oklahoma, Missouri, and Kansas’s borders all meet. This small area of the Ozark Aquifer, which covers almost the entire state of Missouri, encompasses two areas of the aquifer, the Springfield Plateau Aquifer and Ozark Confining areas (Pugh & Adamski, 2003). Figure 1 below shows a map of the different aquifers and their specific locations. Hydrological Setting The area of study projected below in figure 1 is the Western Interior Plains confining systems and the Springfield Plateau aquifer. This part of the Ozark Aquifer is in the Tri-State mining area. The Tri-State area was a mining district in the past for lead and zinc minerals and for strip-mining for coal as well. The average annual rainfall in this area is about 46 inches per year (Miller & Appel, 1997). The evapotranspiration rates vary across the entire aquifer from very high to low from west to east. Overall average across the area is medium to high at around 37 inches per year in the Tri-state region (Miller & Appel, 1997). The annual air temperature is about 56.7 degrees Fahrenheit (Miller & Appel, 1997). The area is in a largely diverse topographic region on the boundary of the Ozark Plateau and the Lowland Plains. The above sea level elevations are quite different throughout the area. The area is located at the base of the Ozark Mountains and can be very hilly with large elevation gains over a few miles. Near Pittsburg, Kansas, the average elevation is 947ft, but in the Miami, OK region, it is closer to 800ft. The highest average elevation in the study area is most likely the Joplin, MO area with an average elevation of just over 1,000ft above sea level (Miller & Appel, 1997). The recharge of the Ozark Aquifer system is mainly from precipitation in the aquifer outcrop areas. It occurs in the Northern part of Arkansas and in the Southwestern part of Missouri. (Figure 1) Figure 1 Retrieved with permission from USGS There are also limited amounts of vertical recharge from the much shallower Springfield Plateau Aquifer into the Ozark Aquifer. The Springfield Plateau aquifer sits on top of the Ozark Aquifer. (Figure 2) Fractures and limited permeability allow water from the Springfield Plateau to enter the Ozark Aquifer vertically (Miller & Appel, 1997). Figure 2 Retrieved with permission from KGS Most of the ground-water flow is controlled topographically from high recharge areas along short flow paths, and is discharged as base flow in nearby streams. The ground water resides and flows through the fractures and in between bedding planes in the carbonate rock that exist in the aquifer. The fractures and bedding planes are significantly enlarged over time do to the dissolution of the carbonate rocks (Miller & Appel, 1997). The runoff in this regional area is around 8-10 inches per year. This runoff makes its way into the local streambeds and rivers and nearby lakes. The limestone rock that is abundant in the area has great permeability allowing ground water to move downward towards the aquifer. This provides a good recharge system for the aquifer. Geologic Properties of the Aquifer Many geological formations comprise this aquifer. They range from Ordovician to Pennsylvanian in age. (Figure3) The Ozark Aquifer is a carbonate aquifer composed largely of limestone and dolomite, although sandstone chert and shale are interbedded at some depths. The main water-yielding formations are the Upper Cambrian Potosi Dolomite, the Lower Ordovician Gasconade Dolomite, and Roubidoux Formation. The aquifer ranges from less than 1,000 to over 3,000 feet in thickness (Miller & Appel, 1997). (Figure3) Figure 3 Retrieved with permission from DNR In the area at its maximum, the Springfield Plateau aquifer is in between 0-450ft in thickness. The rocks are much different in the Plateau Aquifer and in the confining units than in the large Ozark Aquifer. The Ozark Aquifer has much thinner beds of limestone and has some thin shale beds. The confining boundary of the aquifer in all areas of the region that include the Ozark Aquifer, the Springfield Plateau, and the confining units, is a Pre-Cambrian age igneous and metamorphic rock combination with low porosity and permeability. This creates a great lower confining boundary with very limited infiltration from the top or bottom (Pugh & Adamski, 2003). The rocks in all three parts of the aquifer all contain great porosity for holding high water yields (Pugh & Adamski, 2003). The formations almost all are composed of limestone there for they create good fractures in a block like sequence that forms good permeability for the water to move through the rock sequence. The entire aquifer is composed of sedimentary rocks with great pore space that structure an even, almost homogeneous setting. Hydraulic Properties in Tri-State Area Wells in the Springfield Plateau aquifer and in the confining units in southeast Kansas have low in yields (Pugh & Adamski, 2003). These wells typically can be pumped around 20 gallons per minute. The storativity of the area is near 5.1 trillion gallons of water (Pugh & Adamski, 2003). The hydraulic conductivity is considered quite higher in this area versus the large part of the Ozark Aquifer. This along with thin bedding planes causes the water in Springfield Plateau to average 90ft higher than the Ozark Aquifer. 30-40 million gallons of water a year leaks from the Springfield Plateau Aquifer into the Ozark Aquifer due to the stratification differences of elevation. Water in the Springfield Plateau is typically lower in mineralization when in comparison to the Ozark Aquifer. It is calciumbicarbonate rich water due to its large affiliation and direct contact with the limestone rock prevalent in the area (Pugh & Adamski, 2003). This causes the water confined in the aquifer to be quite hard. Water as a Resource The water in the Tri-State area aquifers are used in a variety of ways. The water can be used to supply communities, rural districts, industry, and a large agriculture area. The aquifer supplies an area of about 8,700 square miles. This includes about 12.5 percent of Missouri, thus causing somewhat of a problem due to its large demand with limited resources. The groundwater level can fluctuate year to year by about 15ft (Pugh & Adamski, 2003). Most of the aquifers municipal supply is to Joplin and Springfield Metropolitan Area. Figure 4 below shows the Springfield Metro area has continued to grow in population the last 100 years. Pittsburg, Kansas, and Miami, Oklahoma are two other small cities in the region that use the aquifer as a water source as well. These four communities alone total more than a half a million people. Much of the ground water is used predominately for agriculture purposes. This causes the water level of the aquifer to fluctuate. In a year with more than average rainfall, less irrigation is used on crops. Less water drawn from the aquifer allows time for recharge, leading to a higher water level throughout the area. Figure 4 Retrieved with permission from FAIR Contamination of the Aquifer by Coal Mining In most places the Ozark Aquifer does not contain harmful contaminates, only naturally occurring minerals. The quality of water is acceptable in this case and is used for many purposes including drinking water. In the Ozark Aquifer, concentrations average less the 1,000 milligrams per liter except in the most western parts and eastern parts (Miller & Appel, 1997). But, the Tri-State Mining area has caused many problems within the aquifer in this region. Concentrations of dissolved solids in this area average between 200-500 milligrams per liter, but were recorded as high as 10,000 in the deeper aquifers (Pope, Mehl, Coiner, 2009). This includes the most southeast part of Kansas, southwest Missouri, northeast Oklahoma, and a small area of Northwest Arkansas. This problem is contributed partially by the coal mining in the area. The coal mines in the area were predominately strip pits. This type of mining was used to retrieve the thin shallow beds of coal. The pits usually only hundred feet wide could be up to a hundred feet deep, thus the strip pits collect water from precipitation and cause runoff. The runoffs from the coal strip pits have many affects on the natural water. The runoff increases conductivity, acidity, and sulfate levels, iron and magnesium concentrations, and lowers the pH levels (Fantz, Heatherly, Yasger, 2010). One of the sources from the coal mines is the gob piles left behind. Gob piles are piles of discarded coal-waste and fractured rock. These piles contain iron pyrite also known as fool’s gold. Pyrite is iron sulfide and when it is exposed to water and oxygen, it goes through a chemical reaction that produces sulfuric acid, iron oxides, and hydroxides (Brosius, 2005). The different oxides and sulfides can have a large impact on the pH level of the water it meets. Sulfuric acid is harmful because it pollutes the water and soils it meets. It is not considered toxic but it can be very corrosive. These two types of pollution from the mines are considered Acid Mine Drainage (AMD). The acidic water not only causes harm and corrosion to the elements it comes in contact with like boats, coverts, piers, pumps, and other metal equipment, it causes the destruction of smaller living organisms and causes the water to be unacceptable for recreational purposes and drinking (Fantz, Heatherly, Yasger, 2010). AMD can enter the environment several different ways. One way is from the draining of the gob piles that expel during large events of precipitation. The flowing water transports large amounts of acid into nearby runoff streams and rivers (McKinley, 2008). The second and possibly most devastating way for ADM to effect the aquifer is when in enters vertically underground below the piles. This contaminates large amounts of water once it enters into the aquifer because of how shallow the coal beds are, and many of them directly over lay the aquifer. Any of the acidic runoff trapped in ponds, lakes, or any other holding body of water eventually can leach into the groundwater supply as well (McKinley, 2008). Coalmines or strip pits also cause pollution from one aquifer to another. This is by the mining efforts of the past that actually connect different aquifers to each other by piercing principal confining layers. They can even provide direct pathways from surface to groundwater. This is very harmful to the aquifer because it creates a direct route for surface contaminates to reach the ground water supply without passing through a protective filter of unsaturated zone (Buchanan & Buddemeier, 1993). Contamination of the Aquifer by CBM Production Another source of groundwater contamination in the Tri-State area is through the presence and production of Coal Bed Methanes (CBM). CBM is a gas that is generated during the coalification process and is sorted with the coal on internal surfaces. There are different environmental issues that come into play with the production of CBM. “Environmental groups believe that the extraction, production, and distribution of CBM can have severe impacts on rural agricultural communities” (Fisher, 2001). CBM can development can affect all aspects of the ecological system, including, land, water, wildlife, and communities. One of the issues with CBM production is the large amounts of water that are accompanied with it. When CBM is produced out of the ground, the large amounts of water existing with it come from two places. One of these is that CBM is developed in aqueous solutions. Without the presence of water, CBM cannot be created. This makes producing CBM without producing water nearly impossible. Another object that makes producing CBM without water nearly impossible, is the coal beds in place have a significant amount of porosity. This makes the coal beds ideal for holding large gas reserves (Fisher, 2001). Many times injection wells must be set up to push the gas towards the pumping wellhead. The large amounts of water that are produced along side with the CBM create a significant disposal problem. The surface discharge of saline produced waters or produced waters contain vast amounts of organics or inorganic toxins. These toxins include ammonia or hydrogen sulfide. If insufficient natural flow of water cannot dilute the discharged water on the surface, than there can be substantial, damage possible to the environment (Fisher, 2001). Another source of contamination associated with CBM is involved with the movement and migration of CBM. Methane can move from coal reservoirs to shallow sub-surfaces and or to the ground surface. This is a huge environmental concern as well. Seeping methane can disturb and contaminate shallow groundwater, kill vegetation, and produce fire and explosion hazards within structures, seeping can be prevented through better human practices. Seepage normally happens through natural fractures, in uncemented annular spaces behind existing well casings, through water wells, or through improperly abandoned oil and gas exploration wells (Fisher, 2001). Conclusions and Actions Taken Coal beds in the Tri-State area and other factors, such as the gases they contain, and human influences of mining, cause groundwater pollution in the aquifers in the region. This has been a problem for several years and has been addressed to some extent. There have been new regulations and legislation passed to try and further control the pollution by the governing the production practices of CBM. The two governing bodies that investigate and report on the contamination are both state agencies. In Kansas, the governing body that enforces the clean up is the Kansas Department of Health and Environment (KDHE). In Missouri, the enforcer is the Missouri Department of Natural Resources (DNR). With joint efforts in the region, the two combined have done well at slowing down the pollution factor immensely, but groundwater remediation or clean up is not only difficult but also expensive. In many locations, pollution in the groundwater is known about, but the natural filtration process is relied on to clean it up. In other places, pump stations for municipal water supplies have to include a filtration process to get the water to a good quality level (Fisher, 2001). With new mining and production techniques, and better awareness of the situation, it is hopeful that the water quality can be preserved for our future generations. Resources Brosius, L., (2005, May 4). Coal Mining in Kansas: Kansas Geological Survey Online Publication. Available at http://www.kgs.ku.edu/Extension/cherokee/coalmining.html. Accessed on April 27, 2010. Buchanan, R. and R.W. Buddemeier, (1993). Kansas Ground Water: Kansas Geological Survey Bulletin ED 10. Pgs. 1-5. Available online at http://www.kgs.ku.edu/Publications/Bulletins/ED10/05_qual.html. Accessed on April 25, 2010. Fantz, D.K., W.G. Heatherly, and P.A. Yasger (2010). Watershed Inventory and Assessment: Missouri Department of Conservation Assessment. Pgs. 69-80. Available online at http://extra.mdc.mo.gov/fish/watershed/wosage/watqual/. Accessed on April 29, 2010. Fisher, J.B. (2001). Environmental Issues and Challenges in Coal Bed Methane Production: University of Tulsa Conference Preceedings. Pgs. 2-10. Available online at http://ipec.utulsa.edu/Conf2001/fisher_92.pdf. Accessed on November 3, 2010. McKinley, M.J. (2008). Mining: Pollution Issues. Available online at http://www.pollutionissues.com/Li-Na/Mining.html. Accessed April 29, 2010. Miller, J.A., & Appel, C.L. (1997). Ground Water Atlas of the United States, Kansas, Missouri, and Nebraska: USGS Publication of Ozark Plateaus Aquifer System v. HA 730-D Fgs. 88-113. Available online at http://pubs.usgs.gov/ha/ha730/ch_d/index.html. Accessed April 26, 2010. Miller, J.A., & Appel, C.L. (1997). Ground Water Atlas of the United States, Kansas, Missouri, and Nebraska. USGS Publication of Regional Summary v. HA 730-D Fgs. 1-18. Available online at http://pubs.usgs.gov/ha/ha730/ch_d/index.html. Accessed April 26, 2010. Pope, L.M., H.E. Mehl, and R.L. Coiner. (2009). Quality characteristics of ground water in the Ozark aquifer of northwestern Arkansas, southeastern Kansas, southwestern Missouri, and northeastern Oklahoma, 2006–07: U.S. Geological Survey Scientific Investigations Report 2009–5093. 60 p. Available online at http://pubs.usgs.gov/sir/2009/5093/. Accessed on April 24, 2010. Pugh, A.L., & Adamski, J.C. (2003, August 22). Ozark Plateaus Ground-Water Study. Available online at http://mo.water.usgs.gov/fact_sheets/gndwat.htm. Accessed on April 25, 2010.