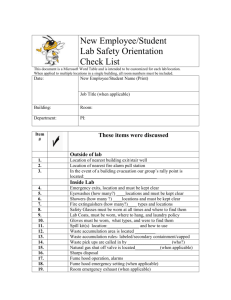
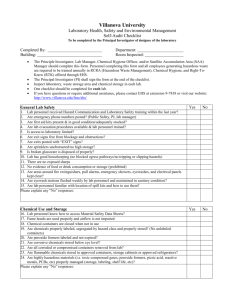
Chemical Hygiene Plan - University of Virginia
advertisement

UNIVERSITY OF VIRGINIA LABORATORY CHEMICAL HYGIENE PLAN TABLE OF CONTENTS Introduction.................................................................................................................................................................................................................. 4 General Laboratory Safety Practices ............................................................................................................................................................................ 5 A. Laboratory Awareness ......................................................................................................................................................................................... 5 B. Personal Safety ..................................................................................................................................................................................................... 5 1. Respiratory and Body Protection ..................................................................................................................................................................... 5 2. Personal Hygiene .............................................................................................................................................................................................. 5 3. Fire Prevention ................................................................................................................................................................................................. 6 D. Housekeeping ...................................................................................................................................................................................................... 6 E. Emergency Procedures ......................................................................................................................................................................................... 6 F. Waste Collection ................................................................................................................................................................................................... 7 G. Children and After Hours Experiments ................................................................................................................................................................ 7 Personal Protective Equipment .................................................................................................................................................................................... 8 A. Eye Protection ...................................................................................................................................................................................................... 8 B. Use and Maintenance of Eyewear........................................................................................................................................................................ 8 C. Corrective and Contact Lenses ............................................................................................................................................................................. 9 1. Corrective Lenses ............................................................................................................................................................................................. 9 2. Contact Lenses.................................................................................................................................................................................................. 9 D. Protective Clothing ............................................................................................................................................................................................... 9 1. Lab Coat ............................................................................................................................................................................................................ 9 2. Apron .............................................................................................................................................................................................................. 10 E. Hand Protection.................................................................................................................................................................................................. 10 F. Foot Protection ................................................................................................................................................................................................... 10 1 The following shoe types should NOT be worn in the laboratory: .................................................................................................................. 11 2. The following are recommended types of footwear: ..................................................................................................................................... 11 G. Ear Protection .................................................................................................................................................................................................... 11 H. Head Protection ................................................................................................................................................................................................. 11 I. Respiratory Protection ........................................................................................................................................................................................ 11 1. Respirator Program ........................................................................................................................................................................................ 12 2. Respirator Types ............................................................................................................................................................................................. 12 Laboratory Safety Equipment..................................................................................................................................................................................... 13 A. Laboratory Chemical Fume Hoods ..................................................................................................................................................................... 13 1. The Control Velocity at the Hood Face ........................................................................................................................................................... 13 2. Air Movement and Flow Patterns in the Room .............................................................................................................................................. 13 3. The Effect of the Operator on the Air Flow Pattern at the Hood Face ........................................................................................................... 13 4. Turbulence within the Hood ........................................................................................................................................................................... 14 1|P a g e 5. Recommended Work Practices ...................................................................................................................................................................... 14 6. Maintenance .................................................................................................................................................................................................. 14 B. Chemical Storage Cabinets ................................................................................................................................................................................. 14 1. Use and Maintenance..................................................................................................................................................................................... 14 2. Types of Cabinets ........................................................................................................................................................................................... 15 C. Individual Storage Containers ............................................................................................................................................................................ 15 D. Refrigerators ...................................................................................................................................................................................................... 15 1. Use and Maintenance..................................................................................................................................................................................... 15 2. Types of Refrigerators .................................................................................................................................................................................... 16 E. Eye Wash Stations .............................................................................................................................................................................................. 16 1. Use and Maintenance..................................................................................................................................................................................... 16 2. Types of Eye Wash Stations ............................................................................................................................................................................ 16 3. Personal Wash Unit (Eye Wash Bottles) ......................................................................................................................................................... 17 F. Safety Showers ................................................................................................................................................................................................... 17 1. Use and Maintenance..................................................................................................................................................................................... 17 2. Types of Safety Showers ................................................................................................................................................................................. 17 G. Fire Safety Equipment ........................................................................................................................................................................................ 18 1. Alarms............................................................................................................................................................................................................. 18 2. FIRE Extinguisher TYPES.................................................................................................................................................................................. 18 3. How to Use a Fire Extinguisher....................................................................................................................................................................... 18 4. Blankets .......................................................................................................................................................................................................... 18 5. Sand/Absorbent Material ............................................................................................................................................................................... 19 6. Sprinklers ........................................................................................................................................................................................................ 19 H. Conclusion .......................................................................................................................................................................................................... 19 First Aid and Emergency Procedures .......................................................................................................................................................................... 20 A. Wounds (Small Cuts, Significant Bleeding & Burns) ........................................................................................................................................... 20 1. Small cuts and scratches ................................................................................................................................................................................ 20 2. Significant bleeding ........................................................................................................................................................................................ 20 3. Thermal Burns ................................................................................................................................................................................................ 20 4. Chemical Burns ............................................................................................................................................................................................... 21 B. Ingestion of Chemicals ....................................................................................................................................................................................... 21 C. Inhalation of Chemicals ...................................................................................................................................................................................... 22 D. First Aid Kits ....................................................................................................................................................................................................... 22 E. Emergency Procedures In Case Of Bodily Injury ................................................................................................................................................. 22 F. Chemical Spills .................................................................................................................................................................................................... 23 1. Acid Spills ........................................................................................................................................................................................................ 24 2. Solvent Spills ................................................................................................................................................................................................... 24 3. Mercury Spills ................................................................................................................................................................................................. 24 G. Fire Safety .......................................................................................................................................................................................................... 24 1. Building or Laboratory Fires ........................................................................................................................................................................... 24 2. Large fires ....................................................................................................................................................................................................... 24 3. Individual on Fire ............................................................................................................................................................................................ 25 h. Conclusion .......................................................................................................................................................................................................... 25 Properties of Hazardous Chemicals ............................................................................................................................................................................ 26 A. Flammability ....................................................................................................................................................................................................... 26 1. Labeling and Information ............................................................................................................................................................................... 26 2. Storage ........................................................................................................................................................................................................... 26 3. Handling ......................................................................................................................................................................................................... 27 B. Corrosivity .......................................................................................................................................................................................................... 27 1. Labeling and Information ............................................................................................................................................................................... 28 2. Storage ........................................................................................................................................................................................................... 28 3. Handling ......................................................................................................................................................................................................... 28 C. Reactivity (Explosives, oxidizers and peroxides) ................................................................................................................................................ 28 1. Explosives ....................................................................................................................................................................................................... 28 a. Labeling and Information ............................................................................................................................................................................... 29 b. Storage and Handling (Explosion hazards, AVOID the following) .................................................................................................................. 29 2|P a g e c. Carefully plan procedures when working with explosive chemicals .............................................................................................................. 29 2. Oxidizers ......................................................................................................................................................................................................... 30 3. Peroxides ........................................................................................................................................................................................................ 30 a. Labeling and Information ............................................................................................................................................................................... 30 b. Storage and Handling ..................................................................................................................................................................................... 31 D. Toxicity ............................................................................................................................................................................................................... 31 1. LD50 (lethal dose 50) ....................................................................................................................................................................................... 32 2. TWA (Time-weighted average) ....................................................................................................................................................................... 32 3. STEL (short-term exclusion limit).................................................................................................................................................................... 32 E. Poisons................................................................................................................................................................................................................ 32 1. Labeling and Information ............................................................................................................................................................................... 33 2. Storage and Handling ..................................................................................................................................................................................... 33 Special Classes of Materials........................................................................................................................................................................................ 34 A. Carcinogens ........................................................................................................................................................................................................ 34 1. Labeling and Information ............................................................................................................................................................................... 34 2. Storage and Handling ..................................................................................................................................................................................... 35 B. Mutagens & Teratogens ..................................................................................................................................................................................... 35 1. Handling and Storage ..................................................................................................................................................................................... 36 C. Compressed Gases ............................................................................................................................................................................................. 37 1. Labeling and Information ............................................................................................................................................................................... 37 2. Storage and Handling ..................................................................................................................................................................................... 37 D. Cryogenic Materials ........................................................................................................................................................................................... 38 1. Storage and Handling ..................................................................................................................................................................................... 38 Chemical Labeling ....................................................................................................................................................................................................... 39 A. Proper Chemical Labeling ................................................................................................................................................................................... 39 B. Hazard Information ............................................................................................................................................................................................ 39 C. Picture Hazard Warnings .................................................................................................................................................................................... 39 D. Symbol Hazard Warnings ................................................................................................................................................................................... 40 E. Word Hazard Warnings ...................................................................................................................................................................................... 40 Material Safety Data Sheets ....................................................................................................................................................................................... 41 Information and Training (HAZARD COMMUNICATION SIGNS) ................................................................................................................................. 42 Chemical Waste Collection ......................................................................................................................................................................................... 43 A. Waste Segregation ............................................................................................................................................................................................. 43 B. Containers and Labels for Hazardous Chemical Waste ...................................................................................................................................... 44 C. Information that is absolutely required on the chemical WASTE DISPOSAL LABEL ........................................................................................... 45 D. Potential Accidents ............................................................................................................................................................................................ 45 E. How to Request a Chemical Waste Pick-up ........................................................................................................................................................ 45 F. Hazardous Waste Sticker .................................................................................................................................................................................... 46 G. Waste Disposal Label ......................................................................................................................................................................................... 46 Glossary ...................................................................................................................................................................................................................... 47 References .................................................................................................................................................................................................................. 52 3|P a g e INTRODUCTION The OSHA Hazard chemical hygiene Standard was promulgated to ensure that all chemicals would be evaluated and that information regarding the hazards would be communicated to employers and employees. The goal of this standard is to reduce the number of chemically related occupational illnesses and injuries. In compliance with the chemical hygiene standard (29 CFR 1910.1450), the scope of this written program applies to University of Virginia faculty, staff and students who work in University of Virginia laboratories. In this document the University of Virginia (UVa) is defined as: UVa (agency 207 and agency 209) laboratory facilities located on the main grounds in Charlottesville, Virginia. Other University of Virginia facilities including non-laboratory facilities and satellite facilities will have their own separate written plans on file at their respective locations to comply with 29 CFR 1910.1450. Copies of this written program are readily available for review by any UVa faculty, staff or student member and can be obtained from the following locations: Office of Environmental Health & Safety (EHS, 982-4911) Office of Environmental Health & Safety web site: (http://ehs.virginia.edu/chemical) Most recent document update: Thursday, March 01, 2007 Updated by: Vaughn C. Kowahl 4|P a g e GENERAL LABORATORY SAFETY PRACTICES The purpose of this written program is to promote safety awareness and encourage safe work practices in the laboratory. These are only guidelines. While they are not rules that will be stringently enforced, they should serve as a reminder of things you can do to work more safely. Although these guidelines are applicable to all research, teaching, and academic laboratories, your lab may require more specialized rules that apply to specific materials and equipment. A. LABORATORY AW ARENESS Label all storage areas, refrigerators, cabinets, etc., appropriately and keep all chemicals in properly labeled (noting date of receipt or generation and the date of the opening of the chemical) container(s). Be alert to unsafe conditions and actions, and call attention to them so that corrections can be made as soon as possible. Pour more concentrated solutions into less concentrated solutions to avoid violent reactions. Be familiar with the appropriate measures you should take when you or someone in your lab is working with or is exposed to the following: CORROSIVE CHEMICALS RADIOACTIVE MATERIALS CARCINOGENS BIOHAZARDS COMPRESSED GASES TOXIC CHEMICALS REACTIVE CHEMICALS FLAMMABLE SUBSTANCES B. PERSONAL SAFETY 1. RESPIRATORY AND BODY PROTECTION Use fume hoods whenever possible. Safety goggles/glasses with side shields should be worn at all times in the laboratory. Laboratory coat/apron should be worn in the laboratory. Gloves should be worn as needed. 2. PERSONAL HYGIENE Wash hands before leaving laboratory. Launder clothing worn in laboratory separately from other clothing. Never use your mouth to pipette chemicals. Avoid having long hair, loose sleeves/cuffs, rings, bracelets, etc. in close proximity to open flames or operating electrical machinery. Keep exposed skin covered. Shorts, skirts, or open-toed shoes should not be worn in the laboratory. 5|P a g e 3. FIRE PREVENTION Be aware of ignition sources in your laboratory area (e.g. open flames, heat, and electrical equipment). Purchase and store flammable reagents in the smallest quantities possible. Do not store flammable liquids in standard refrigerators (an explosion-proof refrigerator should be used). Store flammable liquids in appropriate safety cabinets and/or safety cans. Do not store incompatible reagents together (e.g., acids with flammables). Lists of incompatible reagents can be found in several source books (for example, Handbook of Reactive Chemical Hazards). Do not store ethers for extended periods of time as explosive peroxides could form. Make sure that all electrical cords are in good condition. All electrical outlets should be grounded and should accommodate a 3-pronged plug. D. HOUSEKEEPING Eliminate safety hazards by maintaining laboratory work areas in a good state of order. All equipment should be inspected before use. Use borosilicate glassware for laboratory work. If dichromate/sulfuric acid glass cleaner is used in your laboratory, make sure that cleaning is confined to the fume hood (toxic chromyl chlorides are released from the dichromate/sulfuric acid solution). Better yet, switch to using a non-chromate containing cleaning solution (e.g. NoChromix). If experiments are to be continued unattended overnight, place a note next to experimental apparatus indicating the chemicals involved, your name, and a number where you can be reached in case of an emergency. Keep the laboratory floor dry at all times. Immediately attend to spills of chemicals/water and notify other lab workers of potential slipping hazards. All machinery under repair and adjustment should be properly locked out and tagged prior to servicing. All service work should be done by authorized personnel. E. EMERGENCY PROCEDURES In the event of an emergency, remember one number: 9-911. By calling this number, a variety of emergency response departments can then be alerted to your situation. Be sure the names and phone numbers of lab personnel to be contacted in an emergency are posted on the outside of the laboratory door(s). Be familiar with the location and use of the following safety devices: Safety Shower Fire Blanket Eye Wash Station Fire Alarm Protective Respiratory Gear 6|P a g e First Aid Kit Fume Hood Spill Cleanup Kit Fire Extinguisher Clean up all small spills immediately. If a large chemical spill occurs that you are unable to clean-up call EHS (982-4911). If volatile, flammable, or toxic materials spill, shut off flames and spark-producing equipment at once. Do not cover windows of laboratory doors, except for special experimental requirements. This allows passers-by to notice if anyone is in need of emergency assistance. F. W ASTE COLLECTION Minimize Wastes at the source by limiting the quantities of materials purchased and used. Segregate and prepare chemical wastes for collection in accordance with the procedures issued by EHS. Deposit all waste in designated containers. There are many different types of containers used at the University of Virginia for the collection of the various wastes. Be able to recognize these containers, and know which ones are appropriate for the wastes you generate. G. CHILDREN AND AFTER HOURS EXPERIMENTS Small children and pets should not be brought into the laboratory. If work is being conducted after hours, let other laboratory personnel know of your presence. Avoid carrying out experimental laboratory work in an unoccupied building. 7|P a g e PERSONAL PROTECTIVE EQUIPMENT An expanding array of federal, state, and local laws and regulations make the protection of worker health and safety a legal requirement, as well as an economic necessity. In the final analysis, personal and laboratory safety can be achieved only by informed, responsible individuals. This section summarizes various forms of personal protective equipment. Based on this information, knowledgeable choices for maximum personal protection in the laboratory can be made. A. EYE PROTECTION Because your eyes successfully navigate treacherous situations every day, it's easy to relax your guard in the laboratory environment. After all, for those people not used to wearing glasses, it can be regarded as a burdensome task to wear unattractive, often restrictive eye wear. However, the chemical laboratory is likely to be the most health-threatening place that you can encounter. Splashing chemicals and flying objects are poised to interact with your eyes at any moment, and your eyes frequently get the short end of the deal. For this reason, eye protection is an important consideration. Virginia OSHA regulations make laboratory supervisors, coordinators, faculty members and research advisors responsible for making sure their laboratory employees (this includes students) wear proper eye protection. This eye wear should offer both front and side protection for laboratory personnel and visitors, and must meet federal and state specifications, call EHS if you have any questions. If you don't have safety glasses, tell your supervisor or contact EHS. B. USE AND MAINTENAN CE OF EYEW EAR Eyewear should be as comfortable as possible, fit snugly over the eyes and around the face, and not interfere with the movement of the wearer. When it is appropriate, signs should be posted outside the door stating that eye protection is required before entering the room. Eye protection should be worn when using: Glassware under reduced pressure Cryogenic Materials Glassware under elevated pressure Explosives Caustics, Irritants or Corrosives Biohazards Radioactive Materials UV Light Toxic Chemicals Carcinogens Flammable Materials Lasers 8|P a g e Eye protection should be worn when performing these machine shop operations: Welding Drilling Sanding/Grinding Sawing Eye safety equipment should be capable of being cleaned and disinfected. Eye protection should always be kept in good condition. C. CORRECTIVE AND CO NTACT LENSES 1. CORRECTIVE LENSES Laboratory workers whose vision requires the use of corrective lenses should wear safety eye protection of one of the following types: Prescription safety glasses with protective lenses. Safety eye wear that can be worn over prescription glasses without disturbing the adjustment of the glasses. 2. CONTACT LENSES Laboratory personnel who must wear contact lenses while performing laboratory work should be aware of the following potential hazards: It is virtually impossible to remove contacts from the eyes following a chemical spill affecting the eye area. Contact lenses will interfere with emergency flushing procedures. Contacts may trap and collect fumes and solid materials on the eyes. If chemicals contact the eye area and the laboratory worker is unconscious, rescue personnel may be unaware that contact lenses are present. Use of contact lenses should be considered carefully, with extra consideration given to choosing eye protection that fits snugly over the eyes and around the face. D. PROTECTIVE CLOTHING 1. LAB COAT The lab coat is designed to protect the clothing and skin from chemicals that may be spilled or splashed. It should always be properly fitted to the wearer and is best if it is knee length. There are several different types of lab coats for different types of protection. Cotton -- protects against flying objects, sharp or rough edges, and is a good fire retardant. Wool -- protects against splashes of molten materials, small quantities of acid, and small flames. 9|P a g e Synthetic Fibers -- protect against sparks, infrared and ultraviolet radiation. However, synthetic fiber lab coats can adversely magnify the effects of some laboratory hazards. For instance, some solvents may dissolve particular classes of synthetic fibers, thereby diminishing the protective ability of the coat. In addition, on contact with flames, some synthetic fibers will melt. This molten material can cause painful skin burns and release irritating fumes. Aluminized and Reflective Clothing -- protect against radiant heat. The construction of the material must also be considered (twill, felt, plain, etc.), as the materials are rated differently by various manufacturers. Lab coats should be made with snaps/fasteners which afford the wearer quick removal in the event of an emergency. 2. APRON An apron provides an alternative to the lab coat. It is usually made of plastic or rubber to protect the wearer against corrosive materials and irritating chemicals. An apron should be worn over garments that cover the arms and body. E. HAND PROTECTION It is a good idea to always get into the habit of wearing protective gloves in the laboratory. Aside from acting as a shield between hands and hazardous materials, some gloves can also absorb perspiration and protect the hands from heat. Certain glove types can dissolve when in contact with solvents, it is important to take extra care in matching the protective glove with the nature of the job. Before use, check to make sure the gloves (especially latex gloves) are in good condition and free from holes, punctures, and tears. Gloves should be selected on the basis of the material being handled and the particular hazard involved. Plastic -- protects against light corrosives and irritants. Latex -- provides light protection against irritants (some people can have an allergic reaction to latex which can lead to a serious medical condition). Natural Rubber -- protects against light corrosive material and electric shock. Neoprene -- for working with solvents, oils, or light corrosive material. Cotton -- absorbs perspiration, keeps objects clean, provides some fire retarding properties. Asbestos -- insulates or resists heat. (NOTE: This material should be labeled with the proper warning sign because it is a known carcinogen.) If your laboratory has asbestos gloves that are in need of disposal, seal gloves in a plastic bag and contact EHS. Zetex -- when handling small burning objects. These are a good replacement for asbestos gloves. Care should be taken when removing gloves. Peel the glove off the hand, starting at the wrist and working toward the fingers. Keep the working surface of the glove from contacting skin during removal. Disposable gloves should be discarded in designated containers (e.g., radioactive or regulated medical waste containers). F. FOOT PROTECTION Foot protection is designed to prevent injury from corrosive chemicals, heavy objects, electrical shock, as well as giving traction on wet floors. If a corrosive chemical or heavy object were to fall on the floor, the most vulnerable portion of the body would be the feet. For this reason, shoes that COMPLETELY COVER AND PROTECT the foot are recommended. For more information, contact EHS at 982-4911. 10 | P a g e Fabric shoes, such as tennis shoes, absorb liquids readily. If chemicals happen to spill on fabric shoes, remove the footwear immediately. When selecting footwear for the lab, choose sturdy leather shoes that cover the foot. These will provide the best protection. Safety Toe Shoes, Rubber Boots or Plastic Shoe Covers can prevent contamination. 1 THE FOLLOWING SHOE TYPES SHOULD NOT BE WORN IN THE LABORATORY: sandals clogs high heels shoes that expose the foot IN ANY WAY 2. THE FOLLOWING ARE RECOMMENDED TYPES OF FOOTWEAR: Safety Toe Shoes (steel-toed) -- protect against crushing injuries caused by impact from any object during work activities (e.g., lifting heavy objects, using power tools, etc.). Treated shoes, Rubber Boots or Plastic Shoe Covers -- protect against corrosive chemicals. Insulated Shoes -- protect against electric shock. Rubber Boots with wooden soles -- provide traction in wet conditions where the possibility of slipping exists. G. EAR PROTECTION The Office of Environmental Health and Safety (EHS) will respond to inquiries regarding noise exposure in the work place. Upon request, the staff of EHS will conduct environmental noise and/or personal exposure dosimetry. Ear protection should be worn where the noise level is above 85 decibels (dB). Areas where excessive noise is present should be posted with signs indicating ear protection is required. Ear protectors should be readily available and composed of rubber or plastic. Types of ear protection include: Ear plugs -- provide basic protection to seal the ear against noise. Ear muffs -- provide extra protection against noise, and are more comfortable than ear plugs. Cotton inserts -- are poor suppressers of noise and should be avoided. H. HEAD PROTECTION Some environments within the University have the potential for falling or flying objects. In these settings, appropriate head protection will protect laboratory workers from impacts, penetration by falling and flying objects, and electric shock and burns. Unrestrained long hair can be hazardous. The use of caps, elastic bands, or hair nets will prevent the hair from coming in contact with instrument/machinery parts or flame-producing sources. I. RESPIRATORY PROTECTION 11 | P a g e Because certain laboratory procedures can produce noxious fumes and contaminants, respiratory protection may be required in your work environment. In fact, lab personnel noting changes in air quality should contact EHS (982-4911) and express their concerns. When engineering controls cannot successfully minimize or eliminate the potentially harmful fumes, a respiratory protection program should be established. While the individual departments are responsible for establishing a respirator program, EHS staff will be glad to help institute this program in your laboratory upon request. If your lab procedures require respiratory protection, contact EHS for assistance. 1. RESPIRATOR PROGRAM A respirator program must cover many issues, including: Medical evaluations. Education and training in the use of respiratory equipment. Proper storage and cleaning practices to ensure optimum protection. Equipment adjustment to assure the user of a proper fit and to maximize protection against fumes and contaminants. 2. RESPIRATOR TYPES There are many respirator types available to laboratory workers. These protective devices range from a disposable dust mask to a self-contained breathing apparatus (SCBA). Further information on specific types may be obtained from EHS staff (982-4911). 12 | P a g e LABORATORY SAFETY EQUIPMENT An expanding array of federal, state, and local laws and regulations make the protection of worker health and safety a legal requirement, as well as an economic necessity. In the final analysis, personal and laboratory safety can be achieved only by informed, responsible individuals. This section summarizes various forms of laboratory safety equipment. Based on this information, knowledgeable choices for maximum personal protection in the laboratory can be made. A. LABORATORY CHEMICAL FUME HOODS Chemical fume hoods capture, contain, and expel emissions generated by hazardous chemicals. In general, it is a good idea to conduct all laboratory chemical experiments in a fume hood. While you may be able to predict the release of undesirable or hazardous effluents, in some laboratory operations surprises can always happen. Therefore, the fume hood offers an extra measure of protection. Before use, check to see that your hood has an inspection tag. This will tell you the date of the most recent hood evaluation. If the fume hood in your lab does not appear to be in good working order (a Kimwipe, held inside the fume hood, can indicate if airflow is present), or if you have any questions, call EHS (982-4911). Certain laboratory procedures may require the use of perchloric acid. The use of this material may cause the formation of explosive perchlorate crystals. Special fume hoods, commonly known as Perchloric Acid Fume Hoods, MUST be used for this purpose. These hoods have self-contained wash-down units to inhibit crystal formation. The purpose of a laboratory fume hood is to prevent the escape of contaminants into the laboratory. This is accomplished by drawing air from the laboratory, past the operator, into the hood. The concentration of the contaminant in the actual breathing zone of the operator must be kept as low as possible. The ability of the hood to provide adequate protection is dependent upon the following prime concerns: 1. THE CONTROL VELOCITY AT THE HOOD FACE Face velocities of 80-100 fpm (feet per minute) will provide adequate containment of laboratory contaminants, if the overall installation can be rated as "good" in the reference to the other listed performance factors (see 2 and 3 below). Control velocities must overcome the particle kinetics of aerosols, the molecular diffusion of gases and vapors, and all other "normal" activities which take place inside and outside of the hood. The vector of the air at the face of the hood must be inward and perpendicular to the face. Flows lower than 80 fpm do not provide the safety factors desired for normal conditions such as operator movements. Flows higher than 100 fpm are not required for "good" laboratory arrangements and do not improve performance for poor arrangements. 2. AIR MOVEMENT AND FLOW PATTERNS IN THE ROOM The effect of air movement within the laboratory on the performance of hoods is directly related to hood location and the influence of air supply systems. Hood locations must be away from doors, windows, and pedestrian traffic. Air from these sources can have velocities several orders of magnitude greater than the hood face velocity, creating the potential for dragout or displacement of contaminated air from the hood. Air from outlets such as ceiling and/or wall diffusers, must either be controlled to assist in the performance of the hood or directed so that the energy is lost before entering the zone of influence. Air from the makeup systems should not exceed 20-25 fpm in the hood face area (measured with the hood exhaust "off"). If these criteria are judged satisfactory, the system then can be considered "good" and the required face velocities of 80-100 fpm are valid. 3. THE EFFECT OF THE OPERATOR ON THE AIR FLOW PATTERN AT THE HOOD FACE The turbulent air patterns resulting from the passage of makeup air around an operator standing in front of a hood, have tremendous effects on the air flow characteristics. Serious losses of contaminants from the hood can occur unless the low pressure area in front of the operator is minimized via the proper use of makeup air and the assurance of sufficient capture velocity at the face of the hood. 13 | P a g e 4. TURBULENCE WITHIN THE HOOD As air enters the hood, it is drawn past equipment and sources of contamination toward the exhaust slots. At an airflow greater than needed to provide a good vector and containment, excessive turbulence can cause a "rolling effect" in the hood chamber. This increases the potential for greater mixing of contaminated air and room air at the face of the hood. Under poor laboratory hood arrangements, greater turbulence can result in excessive spill-out of contaminated air into the room. For this reason, it is obvious that substandard hood operations cannot be upgraded merely by increasing air flow. 5. RECOMMENDED WORK PRACTICES All laboratory workers with access to a laboratory chemical fume hood should be familiar with its use. Always work at least six inches back into the hood (six inches beyond the sash line) keeping the sash line between your body and your work. Set the adjustable baffles on the back wall of the hood so that the one on top is about 3/4 of an inch from the ceiling of the hood compartment, and the lower baffle is fully open. This setting will provide the most uniform flow distribution through the hood face for most laboratory operations. A notable exception is the performance of experiments resulting in the discharge of effluents with high thermal buoyancy. Don't use laboratory chemical fume hoods as chemical storage cabinets -- keep the work surfaces clean and uncluttered. Beware of situations when the hood requires sash position mark to denote a minimally acceptable face velocity. This practice is often necessary because the hood cannot deliver the required velocity in the fully open position. However, any time the sash mark must be posted lower than 18 inches up from the hood work surface, there are probably basic (and possibly critical) deficiencies that should be investigated and rectified. 6. MAINTENANCE Fume hoods should be surveyed on a regular basis by EHS. The hood should always be in good condition and capable of routine use. Any hood or component of ventilation not properly functioning must be taken out of service and clearly tagged. The lab worker should not be able to detect strong odors released from materials in the hood area. If odors are detected, check to make sure that the ventilation fan is turned on. To have the operating condition of a fume hood checked, call EHS. An emergency plan should exist in case of hood ventilation malfunction. All protective clothing should be worn when working with chemicals in the hood. In addition to gloves, safety glasses, and lab coats, a face shield will provide an extra measure of safety from reactive chemicals. Solid objects or materials should not be allowed to enter the exhaust ducts at the rear of the hood, as they can become lodged in the duct or fan. B. CHEMICAL STORAGE CABINETS Storage of flammables and corrosives in the lab should be limited to as small a quantity as possible. They should be stored in ventilated cabinets which meet OSHA 1910.106d and NFPA 30 specifications. These specifications are available from EHS. 1. USE AND MAINTENANCE Chemicals should NEVER be stored in alphabetical order (unless already separated out into compatible groups). This system may contribute to the high probability of incompatible materials being stored next to one another. 14 | P a g e Incompatible reagents should not be stored next to each other. Storage outside the cabinet should be kept to a minimum. The vent cap on chemical storage cabinets should not be removed from its location unless the cabinet is attached to an existing ventilation system. Glass containers should be stored on the bottom shelf of storage cabinets, if possible. 2. TYPES OF CABINETS Flammable liquid cabinets -- are designed for storage of flammable or combustible liquids. Acid/corrosive cabinets -- are designed for corrosion resistance. Bulk storage cabinets -- can be used for storage of flammable and corrosive liquids outside the laboratory setting. C. INDIVIDUAL STORAG E CONTAINERS Selecting the best means of storage for chemical reagents will, to a great extent, depend on that reagent's compatibility with the container. A safety can is an approved container of no more than five gallons (19 liters) capacity. It has a spring-closing lid and spout cover, and is designed to safely relieve pressure buildup within the container. Vent caps may be purchased for original manufacturer's glass containers to help minimize explosion hazards. D. REFRIGERATORS While domestic refrigeration units are appropriate for keeping foods cold, they are not designed to meet the special hazards presented by flammable materials. Therefore, laboratory refrigerators should be carefully selected for specific chemical storage needs. To prevent potential safety hazards, the length of storage of any material should be kept to a minimum. In addition, refrigerators should be periodically inspected. 1. USE AND MAINTENANCE Each refrigerator, freezer, or cooler should be prominently labeled with appropriate hazard signs to indicate whether it is suitable for storing hazardous chemicals. To be on the safe side, label chemical hazard refrigerators with the sign "For Chemical Storage Only. No Food or Drink Allowed." If radioactive materials are to be stored, a refrigerator must be clearly labeled "Caution, Radioactive Material. No Food or Beverages may be stored in this unit." The containers placed in the refrigerator should be completely sealed or capped, securely placed, and permanently labeled. Avoid capping materials with aluminum foil, corks, and glass stoppers. Refrigerators should be frost free to prevent water drainage. 15 | P a g e 2. TYPES OF REFRIGERATORS Because ignitable vapors can build up in refrigerators, it is important to store materials in specially-designed units. These refrigerators will have self-contained electrical elements to avoid spark-induced explosions. Explosion-proof refrigerators are specifically designed for hazardous environments, featuring enclosed motors to eliminate sparking. For more information call EHS (982-4911). E. EYE W ASH STATIONS Eye wash stations provide an effective means of treatment when chemicals come in contact with the eyes. Eye wash stations should be readily available and accessible to: (a) all laboratory personnel and; (b) for personnel working in other environments such as studios, shops and garages, where corrosive chemicals are being handled in a way that can create a splash hazard to the face and eyes. The facility should be clearly marked and in accessible locations no more than 10 seconds or 50 feet away from every lab work station. Laboratory workers should be able to locate the nearest eye wash facility with their eyes closed (eye injuries may involve temporary blindness). Eye injury usually accompanies a skin injury. For this reason, eye wash stations should be located near the safety shower so that eyes and body can be washed. 1. USE AND MAINTENANCE Water/eye solutions should not be directly aimed onto the eyeball, but rather, aimed at the base of the nose. This increases the chance of effectively rinsing the eyes free of chemicals (harsh streams of water may drive particles further into the eyes). Eyelids have to be forcibly opened to ensure effective washing behind the eyelid. Be sure to wash from the nose out to the ear this will avoid washing chemicals back into the eye or into an unaffected eye. Flood eyes and eyelids with water/eye solution for a minimum of 15 minutes. Remove contact lenses as soon as possible to rinse eyes of any harmful chemicals. Cover both of the victim's eyes with clean or sterile gauze. Call Rescue Squad (9-911). Plumbed equipment shall be activated weekly to verify proper operation. Eye wash stations should be inspected annually to assure conformance with ANSI Z358.1 section (6) requirements. Plumbed eye wash stations should have protective covers to protect nozzles from airborne contaminants. 2. TYPES OF EYE WASH STATIONS Gravity Feed Self-Contained -- provides the laboratory worker with emergency eye wash treatment in areas inaccessible to plumbing. Faucet-Mounted -- (pin or push plate activators) provides continuous water flow while freeing hands to open eyelids. It turns a standard faucet into a practical emergency eye wash station. Laboratory Bench -- sprays with a squeeze handle can be installed through the bench top for instant availability. Swivel Eye Wash -- mounts on lab bench or counter top adjacent to a sink. It swivels 90 degrees over the sink for use, or out of the way for storage. 16 | P a g e 3. PERSONAL WASH UNIT (EYE WASH BOTTLES) The first seconds following an eye injury are often critical to keeping eye injury to a minimum. Eye Wash Bottles may be kept in the immediate vicinity of personnel working in a potentially hazardous area. However, eye wash bottles should never replace permanent emergency eye wash facilities when they are required. The main purpose of Eye Wash Bottles is to supply immediate flushing. When this has been done, the injured person should go to an emergency eye wash and flush the eyes for the required 15 minutes. (ANSI Z358.1-2004) The injured person should promptly follow up with a medical evaluation. Eye Wash Bottles must be maintained according to the manufacturer’s instructions and promptly disposed of by the expiration date. F. SAFETY SHOW ERS Safety showers provide an effective means of treatment in the event that chemicals are spilled or splashed onto the skin or clothing. Safety shower facilities should be installed wherever chemicals are present (e.g. acids, alkalis, or other corrosive materials) and must be readily available to all personnel. 1. USE AND MAINTENANCE Safety showers should be in a clearly marked and accessible location. The facility should be no more than 50 feet, or 10 seconds, away from every lab workbench. Laboratory workers should be able to locate the shower(s) with their eyes closed (emergency situations may leave victim temporarily blind). Safety showers are operated by grasping a ring chain or triangular rod. The pull mechanism is designed for people of all heights. It should always be accessible and hang freely. Safety shower should supply a continuous stream of water to cover the entire body. Individuals should remove clothing, including shoes and jewelry, while under an operating shower. Safety showers should be located AWAY from electrical panels or outlets. Plumbed equipment shall be activated weekly to verify proper operation. Safety showers should be inspected annually to assure conformance with ANSI Z358.1 section (4) requirements. If at all possible, safety shower facilities should be installed near appropriate drainage systems. 2. TYPES OF SAFETY SHOWERS Ceiling/Wall Emergency Shower -- provides a continuous water flow and mounts directly to overhead vertical pipes or horizontal wall pipes. Deck-Mounted Drench Hose -- is hand operated for quick spot washing of injuries. Floor-Mounted Emergency Combination -- eye wash/face and body wash mounts directly to horizontal wall pipes. 17 | P a g e G. FIRE SAFETY EQUIP MENT If there are any questions on how to obtain an extinguisher or to have an extinguisher inspected, call EHS at 982-4911. 1. ALARMS Alarms are designed so that all endangered laboratory personnel are alerted. All faculty, staff and students should become familiar with the EXACT LOCATION of the fire alarm stations nearest to their laboratory. 2. FIRE EXTINGUISHER TYPES Extinguishers are classified according to a particular fire type and are given the same letter and symbol classification as that of the fire. TYPE A -- Combustibles wood, cloth, paper, rubber and plastics TYPE B -- Flammable Liquids oil, grease and paint thinners TYPE C -- Energized Electrical Equipment electrophoresis TYPE D -- Combustible Metals (magnesium, titanium, sodium, lithium, potassium) Multipurpose Extinguishers are highly recommended because they are an effective agent against Types A, B, and C fires. Extinguishers should be identified by appropriate signage and securely located on the wall near an exit. All extinguishers should be inspected at least every 12 months for broken seals, damage, low gauge pressure, or improper mounting. Units should be replaced or recharged if they have been used, damaged, or discharged. 3. HOW TO USE A FIRE EXTINGUISHER Fire extinguishers are not designed or intended to extinguish large fires, but if used properly, can control or extinguish a small fire. A small fire is defined as one that could occur in a standard office trash can. When a fire or suspected fire, i.e., smoke, is discovered, the first reaction should always be to activate the fire alarm system, call 911, and evacuate the building according to the evacuation plan. Fire extinguishers are provided in all University buildings and can be used provided the person is properly trained to use the extinguisher. The following are guidelines in making the decision as whether to use the unit, and how to use the extinguisher. 1. PULL THE PIN: Place your hand on the top of the cylinder and pull the pin. This will unlock the handle and allow you to activate the unit. 2. AIM: Point the nozzle of the hose at the base of the fire. 3. SQUEEZE: the handle (lever) releasing the fire fighting agent. 4. SWEEP: the nozzle from side to side over the fire. Keep the nozzle/hose directed at the base of the flame. Empty the fire extinguisher onto the fire. 4. BLANKETS Laboratory personnel are DISCOURAGED from using fire safety blankets as a means to extinguish a fire. 18 | P a g e Fire safety blankets should be used as a means to keep shock victims warm. 5. SAND/ABSORBENT MATERIAL Designed for fast and easy extinguishing of small fires in the laboratory. These materials should be stored in a handy dispenser, appropriately labeled, and used according to the type of fire. Do not use sand buckets as ash trays! 6. SPRINKLERS Sprinklers are automatically activated. Laboratory workers should not attempt to shut off the system. Items in the lab should be stored at least 18 inches away from the sprinkler heads. Items should not hang from the sprinkler heads. Intense heat should not be used near the sprinkler heads. Fire detection systems may be temporarily out of service due to utility work performed by the Physical Plant. H. CONCLUSION On the job, eyes, face, hands, and feet are no match for exploding glassware, toxic fumes, chemicals, or falling objects. All work performed in a laboratory is potentially dangerous; however, there are many things laboratory workers can do to minimize the danger. Using the appropriate personal protective and laboratory safety equipment are important safety precautions that one can take to work safely and effectively. For further information on protective and safety equipment options, please contact the Office of Environmental Health and Safety at 982-4911. 19 | P a g e FIRST AID AND EMERGENCY PROCEDURES The first aid and emergency procedures detailed below could be life saving. Become familiar with the information described below, so that disasters can be speedily contained. It is the responsibility of the injured faculty member, staff member or student to report bodily injury, carcinogenic, mutagenic or teratogenic chemical exposure(s) or property damage. University of Virginia (UVA) faculty, staff or students must complete the Accident Report for Workers' Compensation Claim to report on-the-job injury. For a description of UVA Supervisor's and Employee's Workers' Compensation responsibilities, please contact the Workers' Compensation Division (2-4900) or the Office of Risk Management (4-3055). Non-working students and visitors must report all injuries, exposures to carcinogenic, mutagenic or teratogenic compounds and property damage to the Office of Risk Management using the Insurance Incident Form. Always wash your hands before (if possible) and after giving first aid to avoid the risk of infection and transmission of disease. If possible, use latex gloves (or some equivalent if you have a latex allergy) before giving first aid. A. W OUNDS (SMALL CUTS, SIGNIFICANT BL EEDING & BURNS) 1. SMALL CUTS AND SCRATCHES Cleanse area with soap and water. Place a clean dressing over the wound. 2. SIGNIFICANT BLEEDING Call Rescue Squad (9-911) IMMEDIATELY. Calm and reassure the victim. Lay the victim down. This will reduce the chance of fainting. DO NOT remove any objects impaled in the victim. Put direct pressure on the wound with a sterile bandage or clean cloth. If direct pressure does not control the bleeding, elevate the wound above the heart if possible. If bleeding is severe elevate the victims legs about 12 inches, and cover the victim with a blanket. DO NOT APPLY TORNIQUET. 3. THERMAL BURNS First Aid procedures for first degree burns (e.g., sunburn or mild steam burn, characterized by pain, redness and swelling)are as follows: Run cool water over the area of the burn or soak it in cool water for at least 5 minutes. Cover the burn with a sterile bandage or clean cloth. DO NOT APPLY ANY OINTMENTS, SPRAYS OR SALVES. 20 | P a g e Second and third degree burns are characterized by red or mottled skin with blisters (second degree), white or charred skin (third degree). First aid procedures for second and third degree burns are as follows: If the victim is on fire put the fire out. Call Rescue Squad 9-911. Do not remove any burnt clothing unless it comes off easily. Cover burns with dry sterile or clean bandaging. DO NOT APPLY ANY OINTMENTS, SPRAYS OR SALVES. 4. CHEMICAL BURNS If hazardous chemicals should come into contact with the skin or eyes, follow the first aid procedures below. DO NOT become a victim; wear gloves and safety goggles to protect yourself if you are attempting to assist someone covered in chemical(s). Skin Burns Remove victim's clothes -- don't let modesty stand in the way. Remove victim's shoes -- chemicals may collect in the shoes. Rinse the area with large quantities of water for at least 15 minutes (sink, shower or hose). DO NOT apply burn ointments/spray to affected areas. Cover with dry clean or sterile material. For large affected areas, call Rescue Squad (9-911). Eye Burns Eyelids have to be forcibly opened to ensure effective washing behind the eyelid. Be sure to wash from the nose out to the ear, this will avoid washing chemicals back into the eye or into an unaffected eye. Flood eyes and eyelids with water/eye solution for a minimum of 15 minutes. Remove contact lenses as soon as possible to rinse eyes of any harmful chemicals. Cover both of the victim's eyes with clean or sterile gauze. Call Rescue Squad (9-911). B. INGESTION OF CHEMICALS Call Rescue Squad (9-911) IMMEDIATELY. Call the Poison Center (4-5543). If the victim is unconscious, turn their head or entire body onto their left side. Be prepared to start CPR, but be cautious about exposing yourself to chemical poisoning via mouth-to-mouth resuscitation. If available, use a mouth-to-mask resuscitator. 21 | P a g e C. INHALATION OF CHE MICALS Evacuate the area and move the victim into fresh air, if possible. Call the Rescue Squad (9-911). If the victim is not breathing, perform CPR until the rescue squad arrives. Be careful to avoid exposure to chemical poisoning via mouth-to-mouth resuscitation. If available, use a mouth-to-mask resuscitator. If breathing, loosen victim's clothing and maintain the airway. Lay victim flat on their back. Place one hand under their neck and lift. With the heel of other hand on victim's forehead, rotate or tilt head backward into maximum extension. If additional airway opening is required, it can be achieved by thrusting the lower jaw into a jutting-out position. Treat for chemical burns of the eyes and skin. D. FIRST AID KITS First aid kits should be standard equipment in every laboratory. Commercial, cabinet-type, or unit-type first aid kits are acceptable. A typical model for laboratories includes a variety of items specially selected to carry out emergency treatment of cuts, burns, eye injuries, or sudden illness. The first aid kit should contain individually sealed packages for each type of item. Contents of the kit should be checked weekly to ensure that expended items are replaced. Laboratory supervisors are responsible for maintaining the contents of the first aid kit. No oral medication should be dispensed from the first aid kit. E. EMERGENCY PROCEDURES IN CASE OF BODILY INJURY In the event of a chemical exposure, the injured person must seek medical attention in one of the following places (depending on the severity of the exposure): 1. 2. 3. 4. UVa WorkMed (Occupational Health, 545 Ray C. Hunt Dr., Fontaine) for faculty or staff UVa Student Health for students UVa Emergency Room (if after hours or high degree of injury) 9-911 (if injured person cannot move or be moved) 22 | P a g e F. CHEMICAL SPILLS The procedures described below are to be used for chemical spills of 1 - 2 pints. For spills greater than 2 pints, notify supervisor and call either: Please call ENVIRONMENTAL SERVICES (2-1555) if you require assistance in cleaning up a chemical spill and the spill is located at any of the following locations. Barringer Wing Hospital Links Primary Care Center Blue Ridge Hospital McIntire Wing School of Nursing Garage Central Wing McKim Hall Stacey Hall Clinical Department Wing McKim Trailers X-Ray Wing Stacey Hall MRI Corner Building Hospital Links Steele Wing Davis Wing McIntire Wing Suhling Research Lab East Parking Garage Medical School Building University Hospital Elson Student Health Center Moser Radiation Therapy West Parking Garage Finance Annex Multistory Building (OMS) Stacey Hall MRI Health Sciences Library North Wing Steele Wing Please call the OFFICE OF ENVIRONMENTAL HEALTH & SAFETY (982-4911) if you require assistance in cleaning up a chemical spill and the spill is located at any other location. Locate spill cleanup kits. Laboratories should be equipped with spill cleanup kits. If your laboratory area does not have such emergency items, the supervisor can contact EHS, and we can provide you with a free cleanup kit. Wear the appropriate personal protective equipment (e.g., gloves, goggles) when cleaning up spills. 23 | P a g e 1. ACID SPILLS Apply neutralizer (or sodium bicarbonate) to perimeter of spill. Mix thoroughly until fizzing and evolution of gas ceases. NOTE: It may be necessary to add water to the mixture to complete the reaction. Neutralizer has a tendency to absorb acid before fully neutralizing it. Check mixture with Congo Red indicator paper. The red color indicates that the acid has been neutralized. Transfer the mixture to a plastic bag, tie shut, fill out a waste label, and place in a fume hood. Notify supervisor or call EHS for disposal. 2. SOLVENT SPILLS Apply activated charcoal to the perimeter of the spill. Mix thoroughly until material is dry and no evidence of solvent remains. Transfer absorbed solvent to a plastic bag, tie shut, fill out and attach a waste label, and place in a fume hood. Notify supervisor or call EHS for disposal. 3. MERCURY SPILLS Dampen the mercury sponge with water, then wipe the contaminated area. Do this procedure slowly to allow for complete absorption of all free mercury. A silvery surface will form on the sponge. Place the contaminated sponge in its plastic bag, tie shut, fill out and attach a waste label, and place in a fume hood. Notify supervisor or call EHS for disposal. G. FIRE SAFETY 1. BUILDING OR LABORATORY FIRES Small fires (Extinguishable within 1 - 2 minutes) Cover fire with an inverted beaker or wet paper towels. If this fails to extinguish the fire, use a fire extinguisher. To use fire extinguisher: Think P-A-S-S P - pull the pin A - aim the hose at the base of the fire S - squeeze the handle S - sweep the hose back and forth 2. LARGE FIRES REMAIN CALM. Activate manual pull alarm. Call Fire Department (9-911). 24 | P a g e Close the door behind you as you exit the room on your way out of the building. Evacuate by the stairwell, NOT the elevator, assist the injured. Exit building as quickly as possible. 3. INDIVIDUAL ON FIRE Rescuer should have victim: STOP ---- DROP ---- ROLL Fire blankets should not be used to extinguish flames. Call Rescue Squad and Fire Department (9-911). H. CONCLUSION There is always an obligation to assist an injured or ill individual. Application of simple first aid techniques will not only provide you with the knowledge and skill necessary to give life support and other emergency care, but it also will help develop safety awareness habits that promote general laboratory safety. In case you or a lab coworker is injured in an accident, remember one phone number: 9-911. Many emergency agencies can be alerted with this one timesaving (and life-saving) call. In a fire situation, TIME IS OF THE ESSENCE. Be familiar with your Emergency Action Plan and be prepared to act quickly, as you may be able to contain small fires. But remember it is not your job to fight fires. Don't endanger your life. Call a well-trained professional fire fighter (9-911), inform everyone of the danger, and get out of the building quickly. 25 | P a g e PROPERTIES OF HAZARDOUS CHEMICALS A. FLAMMABILITY Flammability is a measure of how easily a gas, liquid, or solid will ignite and how quickly the flame, once started, will spread. The more readily ignition occurs, the more flammable the material. Flammable liquids themselves are not flammable; rather, the vapors from the liquids are combustible. There are two physical properties of a material which indicate its flammability: flash point and volatility (boiling point). The flash point of a material is the temperature at which a liquid (or volatile solid) gives off vapor in quantities significant enough to form an ignitable mixture with air. Given an external source of ignition (i.e., spark, flame), a material can ignite at temperatures at or above its flash point. The flash point of ethyl ether, a highly flammable solvent, is -49 °F; kerosene has a flash point between 100 and 150 °F. Flammable gases have no flash point, since they are already in the vapor phase. The volatility of a material is an indication of how easily the liquid or solid will pass into the vapor stage. Volatility is measured by the boiling point of the material -- the temperature at which the vapor pressure of the material is equal to the atmospheric pressure. The term volatility is often mistakenly used as a synonym for flammability. There are some materials that are volatile but not flammable, such as water, chloroform, and mercury. Some materials are pyrophoric, meaning that they can ignite spontaneously with no external source of ignition. Potassium metal, for example, can react with the moisture in air. This reaction causes hydrogen gas to be evolved, and the heat generated by the reaction can be hot enough to ignite the hydrogen. Examples of commonly-used flammable chemicals: acetone ethyl ether sodium hydrogen lithium acetylene ethyl alcohol potassium 1. LABELING AND INFORMATION Each container of flammable liquid should be properly labeled before use. The label indicating flammability is represented by a flame. Flammability information can be found in the Material Safety Data Sheet under Fire and Explosion Data. Flash point and boiling point information can be found in the section entitled Physical Properties. 2. STORAGE Flammable materials should never be stored near acids. Storage areas should be cool enough to prevent ignition in the event that vapors mix with air. Adequate ventilation should be provided to prevent vapor build up. 26 | P a g e Avoid storage of flammable materials in conventional (non-explosion proof) refrigerators. Sparks generated by internal lights or thermostats may ignite flammable material inside the refrigerator, causing an extremely dangerous explosion hazard. Storage areas should have spill cleanup materials and proper firefighting equipment nearby. Portable fire extinguishing equipment should include dry chemical, foam, or carbon dioxide extinguishers. Storage areas should be inspected periodically for deficiencies, and storage of flammable materials should be kept to a minimum. NO SMOKING signs should be clearly posted where flammable materials are used and stored. Flammable liquids can be separated into categories based on their flash point and boiling point. Based on these classifications, OSHA has published permissible limits for specific flammable liquid storage containers (see table below). *U.L. Approved Plastic Safety Can 3. HANDLING Use gloves and safety goggles when handling flammable liquids or vapors. Dispensing of flammable or combustible liquids should only be carried out under a fume hood or in an approved storage room. When transferring or using a flammable liquid, all ignition sources should be eliminated from the area. Open flames or hot plates should NOT be used to directly heat flammable liquids. DO NOT use water to clean up flammable liquid spills. DO NOT dispose flammable or combustible liquids in the sink or drain. Follow collection procedures issued by EHS. B. CORROSIVITY Gases, liquids, and solids can exhibit the hazardous property of corrosivity. Corrosive chemicals can burn, irritate, or destructively attack living tissue. When inhaled or ingested, lung and stomach tissue are affected. Materials with corrosive properties can be either acidic (low pH) or basic (high pH). Corrosive gases -- are readily absorbed into the body through skin contact and inhalation. Corrosive liquids -- are frequently used in the laboratory and have a high potential to cause external injury to the body. Corrosive solids -- cause delayed injury. Because corrosive solids dissolve rapidly in moisture on the skin and in the respiratory system, the effects of corrosive solids depend largely on the duration of contact. 27 | P a g e Examples of commonly-used corrosives: sulfuric acid hydrochloric acid nitric acid ammonium hydroxide sodium hydroxide chromium trioxide 1. LABELING AND INFORMATION The corrosive label depicts the corrosion of a hand and/or a bar of steel. Information on corrosivity can be found in the Material Safety Data Sheet under Health Effects and First Aid. 2. STORAGE Segregate acids from bases, and corrosive materials from both organic and flammable materials. Store corrosive materials near the floor to minimize the danger of falling from shelves. Store in cool, dry, well-ventilated areas, away from sunlight. The storage area should not be subject to rapid temperature changes. 3. HANDLING Wear adequate protective equipment (lab apron, rubber gloves and splash-proof eye protection). If splashing is a definite hazard, face shields must also be worn. Corrosive materials should be handled in a fume hood to protect from the possible generation of hazardous or noxious fumes. Add reagents slowly. Always add acids to water (never water to acid). During the addition of reagents, allow acid to run down the side of the container and mix slowly. Corrosive materials should be transported in unbreakable containers. C. REACTIVITY (EXPLOSIVES, OXIDIZERS AND PEROXIDES) 1. EXPLOSIVES Explosive materials are chemicals that cause a sudden, almost instantaneous release of large or small amounts of pressure, gas, and heat when subjected to sudden shock, pressure, or high temperature. Some substances, under certain conditions of shock, temperature, or chemical reaction, can explode violently. Such explosions present many hazards to laboratory personnel. Flying glass can seriously lacerate skin. Fires can result from burning gases. 28 | P a g e Corrosive or toxic substances can be liberated. Before working with explosive materials, understand their chemical properties, know the products of side reactions, the incompatibility of certain chemicals, and monitor possible environmental catalysts (such as temperature changes). Examples of commonly-used explosive chemicals: acetylene azide hydrogen nitrogen containing compounds ammonia halogens oxygen perchlorates A. LABELING AND INFORMATION Information on explosives can be found in the Material Safety Data Sheet under Fire and Explosion Data. B. STORAGE AND HANDLING (EXPLOSION HAZARDS, AVOID THE FOLLOWING) allowing picric acid to dry out mixing flammable chemicals with oxidants flammable gas leaks heating compressed or liquefied gas uncontrollable fluctuating temperatures during experiments using explosive chemicals bringing hot liquid (i.e., oil) into sudden contact with a material possessing a lower boiling point contacting flammable materials with catalysts (i.e., acids or bases catalyze an explosive polymerization of acrolein) explosive peroxide decomposition products from building up in solvent containers during storage mixing nitric acid with acetone distilling ethers unless free from peroxides C. CAREFULLY PLAN PROCEDURES WHEN WORKING WITH EXPLOSIVE CHEMICALS Insert experimental apparatus into a dry glove box or gas blanket. Use paper or plastic screw caps on peroxide containers, as friction created by metal screw caps could detonate peroxides. Minimize storage of ethers. Keep specified fire extinguishing equipment near the explosive chemical work space. Determine all explosive hazards prior to experimental work, including the stability of reactants/products. 29 | P a g e 2. OXIDIZERS An oxidizing agent is a chemical used to provide oxygen for chemical reactions. Oxidizers spontaneously evolve oxygen at room or slightly elevated temperatures, and can explode violently when shocked or heated. Because they possess varying degrees of chemical instability, oxidizing agents are explosively unpredictable and, therefore, represent a particularly hazardous safety threat. Examples of oxidizing agents: peroxides hyperperoxides peroxyesters Oxidizers can react violently when in contact with organics. For this reason, avoid interactions between oxidizers and organic materials. Examples of organic-reactive oxidizers include nitric acid, chromic acid, and permanganates. 3. PEROXIDES Some organic compounds, such as ethers, can react with oxygen from the air, forming unstable peroxides. Peroxide formation can occur under conditions of normal storage, when compounds become concentrated by evaporation, or when mixed with other compounds. The accumulated peroxides can then violently explode when exposed to shock, friction, or heat. Pure compounds will accumulate peroxides more readily than compounds containing impurities. Examples of organic compounds that form hazardous peroxides: aldehydes, ketones, ethers compounds with allylene (CH2 = CHCH2R) structure alkali metals, alkoxides, amines vinyl and vinylidene compounds compounds with benzylic hydrogen atoms Examples of chemicals which form hazardous peroxides during exposure to air: cyclohexane tetrahydrofuran (THF) decalin ethyl ether tetralin isopropyl ether A. LABELING AND INFORMATION The oxidizer label depicts a flaming letter "O" on a yellow background. Information on oxidizing agents can be found in the MSDS under the heading Reactivity Data. 30 | P a g e B. STORAGE AND HANDLING Discard opened containers of peroxidizable compounds after 1 month. Discard unopened containers of peroxidizable compounds after 12 months. For disposal, call EHS (982-4911). Order ether in small quantities and use quickly. Include the date of purchase on containers of peroxidizable compounds. After opening, note the date of use on the label. When possible, store peroxidizable compounds (except certain inhibited vinyl monomers) under a nitrogen atmosphere. Keep away from heat, light, and ignition sources. Store in a cool, dry, well-ventilated area, out of direct sunlight. Protect from extreme temperatures and rapid temperature changes. DO NOT SMOKE near oxidizers. Store in amber glass or inert containers, preferably unbreakable. Containers should be tightly sealed, and stored in an area with good ventilation. DO NOT use corks or rubber stoppers to cap containers. Before opening glass bottles, look for the presence of solids (crystals) or viscous liquid at the bottom of the bottle. These are good indicators of peroxide formation. If either are present -- Do not open container -- call EHS for disposal (982-4911). Isolate reactive chemicals from: organic materials flammable solvents corrosives (i.e., nitric, chromic acids) toxicants Avoid friction, grinding, and all forms of impact while working with oxidizers. Avoid mixing oxidizing agents with other chemicals during disposal procedures. To detect the presence of peroxides, the following procedure can be used. In a 25ml glass-stoppered cylinder (colorless, protected from the light), add 1 ml of freshly prepared 10% aqueous potassium iodide solution to 10 ml of organic solvent. View the cylinder transversely against a white background. If a yellow or brown color appears, peroxide is present. Call EHS for disposal (982-4911). Peroxides can be removed from organic compounds by passing the solvent through a column of activated alumina. Call EHS for waste pick-up. D. TOXICITY The concept of toxicity is unique because it can be applicable to all chemical substances used in the laboratory. The terminology explained below can not only assist laboratory workers in assessing the degree of hazard, but it can also provide guidance in the selection of appropriate personal protective equipment. As defined, toxicity is the ability of a substance to cause damage to living tissue, impairment of the central nervous system, severe illness, or in extreme cases, death when ingested, inhaled, or absorbed through the skin. The administration of a particular dosage of a chemical, and the subsequent response by experimental animals, can help predict that chemical's toxic effect on humans. The dose-response behavior is represented by a dose-response curve, which demonstrates that not all individuals will respond to a particular dose of a chemical in the same manner. Some people will be more sensitive than others, and a specific dosage that may be lethal to one person may not be lethal to another. Therefore, an average measure of toxicity must be denoted. 31 | P a g e 1. LD 50 (LETHAL DOSE 50) The point on the curve where 50% of the test animals have died as a result of a particular chemical dosage is referred to as the Lethal Dose50, or LD50. The LD50 is usually indicated in terms of milligrams of substance ingested per kilogram of body weight (mg/kg). The lower the LD50, the more toxic the material. Inhalation of toxic substances can cause a great deal of tissue damage. Each lung is composed of a large surface area of folded tissue, which would be vulnerable to assault by toxic vapors and airborne particles. The toxicity of a substance via inhalation is measured by TLV's, or threshold limit values. These values are determined by the American Conference of Governmental Industrial Hygienists (ACGIH) and are expressed in parts per million (ppm) of the substance in air, or milligrams of substance per cubic meter of air. There are two major types of threshold limit values: the time-weighted average (TWA) and the short-term excursion limit (STEL). The toxicity of a substance via absorption can be determined several ways. Often, the threshold limit values of a substance will have a skin notation, indicating they are rapidly absorbed by the skin. Absorption can also be indicated by the solubility of the material in water. Materials that are extremely soluble in water can dissolve in skin moisture and be transported through the skin's surface. For instance, dimethyl sulfoxide (DMSO) rapidly absorbs into the skin. If any toxic materials are present in this solvent or on the surface of the skin, DMSO will transport these contaminants into the body as well. A substance can have either acute or chronic toxicity. A substance that is acutely toxic will have immediate effects on the health of an over-exposed individual. A substance that has chronic toxicity will eventually affect the health of a person due to long-term exposure to that material. A listing of OSHA-regulated substances and there PEL's (permissible exposure limits), TWA's, and STEL's are contained in OHSA 29 CFR 1910.1000 Table Z-1-A, Z-2, and Z-3. Consult your supervisor or EHS if you have any questions about the chemicals that you work with. Tables Z-1-A, Z-2, and Z-3 are available from EHS (982-4911). 2. TWA (TIME-WEIGHTED AVERAGE) The TWA of a substance is the average concentration to which a worker can be exposed throughout an eight-hour work day without adverse effects. An important point to keep in mind is that the adverse effects of over-exposure to a material can range from headache or nausea to more severe disabilities. For this reason, time-weighted averages should be considered only as a guide in controlling health hazards in the laboratory, not as definitive marks between safe and dangerous concentrations. 3. STEL (SHORT-TERM EXCLUSION LIMIT) The STEL of a substance is the maximum amount to which a worker can be exposed in a fifteen-minute period without adverse effects. Again, this is intended only as a rough guideline. E. POISONS A poisonous compound is a substance that causes death or serious injury in the event that relatively small amounts are inhaled, ingested, or have contacted the skin. All substances can be poisonous in varying quantities (e.g., a little cyanide or a lot of toothpaste). 32 | P a g e 1. LABELING AND INFORMATION Any substance that carries the international poison symbol (skull and crossbones) should be treated as hazardous. Information on the poisonous nature of chemicals can be found in the MSDS section Health Hazard Data. 2. STORAGE AND HANDLING Treat poisonous compounds with extreme caution. Wear protective lab coats, gloves, and safety glasses and work in a wellventilated fume hood. Wash hands frequently. 33 | P a g e SPECIAL CLASSES OF MATERIALS A. CARCINOGENS A carcinogen is an agent capable of causing cancer, as designated by the Occupational Safety and Health Administration (OSHA). The list of designated carcinogenic chemicals is constantly being modified. Long-term exposure to carcinogenic substances can result in cancers of various types. A number of substances have been found to be capable of producing cancer following exposure by inhalation, ingestion, or skin contact. The division director has overall responsibility for ensuring the safe use and proper disposal of carcinogenic chemicals. Employees wishing to work with regulated carcinogens are to notify their division director. In addition, the Occupational Health Department should be provided with a list of employees handling any known carcinogens. 1. LABELING AND INFORMATION The following terms are used to describe carcinogenic materials: Sufficient positive -- Those chemicals that were found to promote and increase incidence of malignant tumor in a multiple species or strain of lab animals. Limited positive -- Those chemicals found to promote either malignant tumors in a single strain, or benign tumors in single or multiple species or strain. Inadequate -- Insufficient evidence to make a decision. Equivocal -- Almost no supporting evidence. Negative -- (limited or sufficient) significant negative evidence. Examples of known or suspected carcinogens are listed below. The risk factor associated with these compounds is high, alternative compounds should be used whenever possible. 4-Nitrobiphenyl and §-Naphthylamine * Methylchloromethyl ether 3,3'-Dichlorobenzidine * bis(chloromethyl) ether * Chloroform * Benzidine * 4-Aminodiphenyl Ethyleneimine * §-Propiolactone Benzene * Dimethylaminoazobenzene Vinyl chloride * 1,2-dibromo-3-chloropropane * Arsenic Acrylonitrile * 34 | P a g e N-Nitrosodimethylamine * Formaldehyde * *Designates a controlled substance (EPA). Entrances into areas where known carcinogens are used in appreciable quantities shall be posted: Cancer Suspect Agent Authorized Personnel Only 2. STORAGE AND HANDLING Containers should be clearly labeled and kept in a separate (preferably locked) storage location. Immediate work areas should be clearly demarcated with warning signs. All work surfaces which are used should be covered with stainless steel, plastic trays or dry absorbent plastic-backed paper. Laboratory supervisors and/or faculty advisors are responsible for training laboratory workers on proper handling techniques. Each laboratory worker must adhere to proper operations, emergency procedures, monitoring of lab work and required medical examinations. Medical records must be accurately maintained when working with carcinogens. Have your supervisor or faculty advisor provide a list of employees and students working with substances labeled as carcinogenic to Occupational Health. If you believe that you have been exposed to a substance labeled as carcinogenic notify your supervisor or faculty advisor, complete an Accident Form for Worker's Compensation Claim Form and inform Occupational Health. Before working with a suspected or known carcinogen obtain health hazard information for each compound. In addition, compile spill cleanup emergency procedures for your laboratory. For more information, call EHS (982-4911). Exercise extreme caution. Wear personal protective clothing and equipment and work in a well ventilated area. Visitors should be notified about carcinogen use in the laboratory work area. B. MUTAGENS & TERATOGENS Mutagens Mutagens are chemical and physical agents that induce mutations in DNA and in living cells. This affects the genetic system in such a way as to cause cancer or hereditary changes in chromosomes. Individuals exposed to chemicals with mutagenic properties may develop genetic damage to the extent that future offspring will be affected. Two forms of somatic (body/organ) cell interference may be noted. Leukemia -- White blood cells are produced far more rapidly than they can be removed from the blood, interfering with normal body functions. Cancer -- Cells that do not normally divide during adult life begin to proliferate to the extent that such division displaces or invades normal tissues. 35 | P a g e Examples of Mutagens: Arsenic Ethidium Bromide Ionizing Radiation (gamma, x-rays) Alkylating agents (e.g., dimethyl sulfate) Teratogens Teratogens are chemical and physical agents that interfere with normal embryonic development. Teratogens differ from mutagens in that there must be a developing fetus. Damage to the fetus (embryo) is most likely to occur early in pregnancy, during the first 8 - 10 weeks. Teratogens may produce congenital malformations or death of the fetus without inducing damage to the pregnant woman. In general, carcinogenic, mutagenic and teratogenic chemicals should be considered as hazards to reproductive health. Even though OSHA has established exposure limits of dangerous materials, a developing fetus may be adversely affected by lower doses than those considered "safe". Toxicology is still not well developed to evaluate reproductive health hazards. As of 1985, OSHA has identified three substances as Teratogens. Dibromochloropropane Lead Ethylene oxide Examples of several other materials that are thought to be associated with reproductive health disorders are listed below. Antimony Carbon disulfide Ethylene thiourea Polychlorinated biphenols (PCBs) Nitrous oxide Formaldehyde Ethylene dibromide Ionizing radiation 1. HANDLING AND STORAGE Containers should be clearly labeled and kept in a separate (preferably locked) storage location. Immediate work areas should be clearly demarcated with warning signs. All work surfaces which are used should be covered with stainless steel, plastic trays or dry absorbent plastic-backed paper. Laboratory supervisors and/or faculty advisors are responsible for training laboratory workers on proper handling techniques. Each laboratory worker must adhere to proper operations, emergency procedures, monitoring of lab work and required medical examinations. Medical records must be accurately maintained when working with mutagens or teratogens. Have your supervisor or faculty advisor provide a list of employees and students working with substances labeled as mutagenic and/or teratogenic to Occupational Health. 36 | P a g e If you believe that you have been exposed to a substance labeled as mutagenic or teratogenic notify your supervisor or faculty advisor, complete an Accident Form for Worker's Compensation Claim Form and inform Occupational Health. Before working with a suspected or known mutagen or teratogen obtain health hazard information for each compound. In addition, compile spill cleanup emergency procedures for your laboratory. For more information, call EHS (982-4911). Exercise extreme caution. Wear personal protective clothing and equipment and work in a well ventilated area. Visitors should be notified about mutagen and teratogen use in the laboratory work area. C. COMPRESSED GASES The purpose of this section is to assist the laboratory worker with identification, storage, maintenance, and handling of compressed gases. Compressed gases can be hazardous because each cylinder contains large amounts of energy and may have high flammability and toxicity potential. 1. LABELING AND INFORMATION Compressed gas containers may be labeled in five ways: 1. Flammable Gas -- labels show a flame on red label. 2. Non-flammable Gas -- labels depict a gas canister on a green background. 3. Poisonous Gas -- labels show a skull and crossbones. 4. Oxygen-containing Gas -- labels are designated by a flaming letter "O". 5. Chlorine Gas -- labels are distinctly marked. Know the contents of the cylinder and be familiar with the properties of the gas. The contents of the cylinder or compressed gas should be clearly marked and identified with proper labels or tags on the shoulder of the cylinder. Those cylinders or compressed gases that do not comply with identification requirements should be returned to the vendor. If two labels are associated with one cylinder, affix the labels 180° apart on the shoulder of each cylinder. Label all empty cylinders EMPTY or MT and date the tag. All regulators, gauges, valves, manifolds, must be designed for the particular pressures and gases involved. They should bear the inspection seal of either Underwriters' Laboratories (UL) or Factory Mutual Engineering Division of Associated Factory Mutual Fire Insurance Companies (FM). 2. STORAGE AND HANDLING All cylinders should be stored in cool, dry, well-ventilated surroundings and away from all flammable substances including oil, greases, and gasoline. DO NOT subject any part of a cylinder to a temperature higher than 125 °F. Cylinders should not be located where objects may strike or fall on them. Cylinders should not be stored in damp areas, or near salt, corrosive chemicals, fumes, heat, or direct sunlight. Store cylinders by gas type, separating oxidizing gases from flammable gases. 37 | P a g e All cylinders and compressed gases (full or empty) should be properly fastened and supported by straps, belts, buckles, or chains to prevent them from falling and causing bodily harm. A maximum of two cylinders per restraint is preferred. DO NOT SMOKE in areas where there are flammable gases being used or stored. DO NOT extinguish a flame caused by a gas until the gas source has been shut off. A cylinder should only be moved while strapped to a wheel cart to ensure stability. When storing or moving cylinders, always attach safety caps. DO NOT heat the cylinder or place a cylinder where it may become part of an electrical circuit. Compressed gases must be handled as high-energy sources and dangerous projectiles. All cylinders should be checked for damage prior to use. DO NOT repair damaged cylinders yourself. Damaged or defective cylinders, valves, etc., must be taken out of use immediately and returned to the manufacturer for repair. Each regulator valve should be inspected annually. Never force valve or regulator connections. Threads and the configuration of valve outlets are different for each family of gases to prevent mixing of incompatible gases. DO NOT use lubrication on valve regulators. D. CRYOGENIC MATERIAL S Cryogenic materials have special properties that make them particularly hazardous to use in the solid, liquid, or gaseous state. 1. STORAGE AND HANDLING The severely cold temperatures associated with cryogenic liquids (-60 °C to -270 °C) can damage living tissue on contact and embrittle structural materials. Liquefied under pressure, cryogenic liquids must be kept in specially designed, high-pressure vessels that contain fittings to relieve pressure. When in contact with a moist area, ice formation can plug pressure release devices and pose an explosion hazard. For this reason, store vessels in a dry place and periodically check for ice formation. Cryogenic liquids present fire and explosion hazards. A flammable mixture, cooled in the presence of air with liquid nitrogen or liquid oxygen, can cause oxygen to condense and thereby present an explosion hazard. Keep away from ignition sources. Flammable liquids will support combustion in both the liquid and gaseous states. If allowed to depressurize, cryogenic liquids will rapidly and violently expand. Store and work with cryogenic liquids in a well-ventilated area. These liquids can cause asphyxiation as evaporating CO 2 is concentrated around cryogenic materials. Safety glasses and face shields should be used. For handling of cryogenic liquids, use potholders instead of gloves (as gloves can freeze to the skin). Cushion glassware in a protective covering to prevent injury caused by flying glass in the event of implosion/explosion. Transport fragile cryogenic containers with caution -- use a hand truck. 38 | P a g e CHEMICAL LABELING Labels will be the primary, initial source of warning for employees when handling hazardous chemical substances. Federal and State regulations mandate that all labels on original/stock containers of hazardous chemicals include the name of the hazardous chemical, appropriate hazard warning(s), and the name and address of the manufacturer, importer, or other responsible party. Substances regulated by a specific OSHA standard must be labeled by the manufacturer according to the requirements of that standard. AN EXAMPLE: OSHA 1910.1018(p) -- The Inorganic Arsenic Standard states that containers of inorganic arsenic must have a label which bears the following information: DANGER CONTAINS INORGANIC ARSENIC CANCER HAZARD HARMFUL IF INHALED OR SWALLOWED USE ONLY WITH ADEQUATE VENTILATION A. PROPER CHEMICAL L ABELING Laboratory supervisors should ensure that all incoming containers of hazardous materials bear a label specifying: Appropriate hazard warnings (i.e., pictures, symbols, words, or any combination thereof which convey the hazard(s) of the chemical(s)). Identification of the chemical in the container and identification of the hazardous component(s). Name, address, and telephone number of the chemical manufacturer, importer, or responsible party (e.g. principle investigator). Laboratory workers should not remove or deface labels on containers of hazardous chemicals. When chemicals are transferred from the manufacturer's original container to a secondary container, that new container should be appropriately labeled as to chemical identity and hazard warning(s). B. HAZARD INFORMATION Hazard warnings found on the labels of hazardous chemical containers may be composed of pictures, symbols, and words, or any combination thereof which convey the hazard(s) of the chemical. C. PICTURE HAZARD W ARNINGS Picture hazard warnings help to identify the properties and classes of hazardous compounds. Examples include the flaming letter "O" (oxidizer), hand/bar of steel (corrosive) and a skull-and-crossbones (poison): 39 | P a g e D. SYMBOL HAZARD W ARNINGS Symbol hazard warnings provide basic information in determining what precautionary measures to use when handling hazardous chemical substances and/or dealing with a fire. E. W ORD HAZARD W ARNI NGS Word hazard warnings contain a word or words intended to capture the worker's immediate attention (e.g. flammable, poison, fatal if swallowed). These word labels should be in English, but other languages may be used where needed. o Signal words - are warnings used to designate the degree of hazard. o DANGER -- Highest degree of hazard (red text) o WARNING -- Intermediate degree of hazard (orange text) o CAUTION -- Lowest degree of hazard (yellow text) 40 | P a g e MATERIAL SAFETY DATA SHEETS Material Safety Data Sheets (MSDS) are the backbone of the UVA Chemical Safety Program. OSHA requires chemical manufacturers and importers to produce one MSDS for each hazardous chemical they manufacture or import. MSDS are maintained at EHS, and will provide more detailed health and property information than is provided on individual container labels. Laboratory supervisors, employees, and students can get a copy of any MSDS either by calling 982-4911, stopping by the EHS office on Edgemont Road and and are also available online at: http://ehs.virginia.edu/MSDS. If you have any emergency questions or concerns, call EHS. MSDS will tell you of any special procedures that may be required for the safe handling of a specific substance. If you are taking any medications, if you are pregnant, or if you have a medical condition such as an allergy talk with your supervisor or physician for specific instructions. When using MSDS you must apply your own good professional judgment to the information that they contain. MSDS include information and procedures that usually only apply to industrial settings, these situations will most likely not occur in the laboratory environment. Even so, MSDS are a valuable source of information when you have questions or concerns about any chemical that you work with. It is a good idea to consult the MSDS for any new chemical that is introduced into your work routine, this is a quick and easy way to familiarize yourself to any hazards or precautions that you should take when working with a new substance. Faculty, staff and students are encouraged to send copies of MSDS that they receive, to the Office of Environmental Health and Safety, so they may be kept on file and available to emergency personnel and others who may require the information! Send copies to P.O. Box 400322 or fax to (434) 982-4915. Material Safety Data Sheets include the following information: The identity of substance designated on the container label Single substance -- chemical and common names Mixtures tested as a whole -- chemical and common names of all ingredients which are health hazards, in concentrations of 1% or greater. Mixtures untested as a whole -- chemical and common names of all ingredients which are health hazards and which are in concentrations of 1% or greater; carcinogens in concentration of 0.1% or greater. Physical and chemical characteristics of the hazardous chemicals. Physical hazards (potential for fire, explosion, etc.). Known acute and chronic health effects and related health information. Primary routes of entry into the body. Information on exposure limits. Whether a hazardous chemical is considered a carcinogen by OSHA, the International Agency for Research on Cancer, or the National Toxicology Program. Precautions for safe handling. Generally acceptable control measures (engineering controls, work practices, personal protective equipment). Emergency and first aid procedures. Date of MSDS preparation, or most recent change. Name, address, and phone number of the party responsible for preparing and distributing the MSDS. A MSDS may be used for similar mixtures with essentially the same hazards and contents. 41 | P a g e INFORMATION AND TRAINING (HAZARD COMMUNICATION SIGNS) In accordance with Federal and State regulations, all personnel (working with or around chemicals) have the "right to be informed and trained" on the chemical hazards present in their work area. The responsibility for apprising workers of the necessary precautions to take when using or handling hazardous materials rests with the Supervisor, Laboratory Manager or Faculty Member in charge. Ultimately your safety depends on you! So take the time to learn about the hazards, the precautions to be taken, and carry out your role safely. If you have questions, ask your Supervisor, Laboratory Manager or Faculty Member, or call EHS (982-4911). All new and returning faculty, staff and students will be provided a brochure titled: OSHA Hazard Communication Standard “Right to Know”. This brochure serves as awareness training. All persons working in chemical laboratories or generating Hazardous (chemical) Waste must also complete, ANNUALLY, the training found here: http://www.ehs.virginia.edu/chemical EHS keeps records of attendance of training sessions presented by EHS staff members and keeps records of training provided through the EHS World Wide Web Site. Supervisors, Laboratory Managers or Faculty Members who provide specific training would be well advised to keep a signed statement from employees/students indicating that they have received the appropriate training. A form which you may use is available from EHS. All rooms/areas where hazardous materials are stored or used will have signs like these (see below) which give hazard (see Symbol Hazard Warnings) and emergency contact information. 42 | P a g e CHEMICAL WASTE COLLECTION The Environmental Protection Agency (EPA) is closely scrutinizing academic institutions these days. Under EPA regulations, those individuals who continue to dump chemical waste down the drain will face stiff fines and a possible jail term. This punishment will be levied against the offending laboratory employee, not the University. All this talk of fines is unnecessary, however, because UVA maintains a user-friendly waste disposal program. The Office of Environmental Health and Safety (EHS) answers waste collection questions and will even pick up your chemical waste upon request, but we need your help. Take the time to follow the simple guidelines below, and call EHS if you have any questions (9824911). A. W ASTE SEGREGATION We prefer to keep certain types of chemicals separated at the time of collection. This method not only lowers disposal costs for the University, but also decreases the chances of incompatible materials being added together. Keep the following groups to themselves whenever possible: Non-halogenated organic solvents, <5% water Non-halogenated organic solvents, >5% water Halogenated solvents (% water unimportant) Solutions containing compounds of the following metals: arsenic, barium, cadmium, chromium, lead, silver and selenium. Any solution containing mercury or its compounds. (Mercury/mercury compounds should be kept separate from any liquid whenever possible.) Acids, organic Acids, mineral Bases, organic Bases, mineral Acyl Halides (e.g. acetyl chloride, thionyl chloride, benzoyl chloride) Cyanides Sulfides Organic peroxides Inorganic Oxidizers Photographic fixer Photographic developer Photographic stop bath Water-reactive compounds (e.g. sodium, butyllithium, grignard reagents) Pesticides Oils Paints Formaldehyde Solutions Do not put acidic or basic waste (pH <3 or >9) in metal cans. Metal cans corrode in a very short time. Keep acids and bases separate from hydrocarbons and ethers. 43 | P a g e When possible, keep all carcinogens/mutagens/teratogens separate from other waste. Keep aqueous wastes separate from organic solvents. Keep halogenated solvents and wastes separate from non-halogenated solvents. B. CONTAINERS AND LABELS FOR HAZARDOUS CHEMICAL W ASTE Do not put hazardous waste down the sink or in the trash. If you are not sure if a chemical is hazardous, call EHS. Chemical Waste must be labeled at all times. EHS provides containers, labels, and chemical waste pick-up forms free-of-charge. EHS provides the following containers for chemical waste collection, activities requiring special containers are evaluated on a caseby-case basis. 4 liter (~1 gallon) plastic-coated glass bottle. 5 gallon plastic carboy. This is the preferred (DOT-approved) container for liquid chemical waste. 5 gallon bucket. The 5 gallon bucket is used for solid and gel waste. Liquid waste should NEVER be put into a bucket like this. Chemically contaminated needles should be placed in Sharps-a-Gator boxes, and will be disposed of by EHS. All chemical waste must be deposited in properly labeled waste containers. According to the Virginia Department of Waste Management, each waste container MUST be marked with a HAZARDOUS WASTE STICKER (see below), issued by EHS. Any containers issued by our office will already contain this sticker. If you plan to use your own bottles as waste receptacles, you can receive the required stickers by contacting our office (982-4911). In addition to waste stickers, all waste containers MUST contain a WASTE DISPOSAL LABEL (see below), issued by EHS. This includes chemicals still in their original containers. Waste will not be picked up if it is not labeled properly. If you need new labels, let us know and we will deliver them on our pickups. 44 | P a g e Both the label and its no-carbon-required copy should be affixed to the waste container by a single piece of tape across the top of the label, or in such a way that we can remove the copy when we pick up the waste. Chemically contaminated lab trash should be collected in trash bags inside 5-gallon screw top pails, also provided by EHS, NOT in CMC's. Only Regulated Medical Waste should go into a CMC. If you have questions about whether your chemically contaminated trash needs to be picked up by EHS call EHS at 982-4911. C. INFORMATION THAT IS ABSOLUTELY REQUIR ED ON THE CHEMICAL W ASTE DISPOSAL LABEL The names of all possible contents, including stains, water, or any solvents. Do not use abbreviations or formulas. The percentages of each component (total must equal 100%). The total quantity. The pH of the waste liquids if it is suspected to be below pH 3 or above pH 10. Also include your name, date, department, building and room number where the waste is located, phone number, and lab director. IMPORTANT: Disposal companies will not accept unknown chemicals. You must make every possible effort to accurately describe the contents of each container. This means tracking down and questioning previous lab occupants if necessary. DO NOT FILL CONTAINERS TO THE TOP. Fill plastic carboys ONLY to the fill line. Leave about 2 inches at the top of all other containers. All waste must reside in closed, non-leaking containers. Do not use flasks or test tubes with stoppers, beakers with parafilm, or bottles with ground glass stoppers. The outside of the waste container must be reasonably clean. Do not put liquids (especially phenol) in bottles designed for solids. They leak! The Virginia Department of Waste Management has stated that all chemical waste containers must remain CLOSED (capped) between chemical waste additions. When chemical waste containers are left uncapped, laboratory personnel are at the risk of chemical exposure due to inhalation of chemical vapors. This will be an area of emphasis in future Virginia Department of Waste Management inspections of the university. We do not pick up empty bottles. They may be triple rinsed and discarded. We will supply empty bottles, as well as 5-gallon cans and carboys. Call in advance for these items and we will bring them with your regular pickup. D. POTENTIAL ACCIDENTS Ethers tend to form extremely explosive compounds over time. Therefore, date all ether cans. Do not keep an open ether can for more than 1 month or an unopened can for more than 12 months. If you have an old ether can, label as waste and call EHS for pick up. Do not attempt to open any bottles of DRY picric acid. This is an extreme explosion hazard!!! Any dry bottles of picric acid should be labeled as waste and picked up by EHS staff. E. HOW TO REQUEST A CHEMICAL W ASTE PICK-UP Do not accumulate more than five 5-gallon cans or carboys, or more than ten gallons in bottles. Larger pickups will have to be scheduled separately. Call 982-4911 or visit ehs.virginia.edu (EHS has an online form) to schedule a waste pick up. Chemical waste will be picked up within three working days from the date it was requested to be picked up. 45 | P a g e F. HAZARDOUS W ASTE STIC KER G. W ASTE DISPOSAL LA BEL 46 | P a g e GLOSSARY Glossary Acid An organic or inorganic compound that has a pH of less than 7. Acidic materials are corrosive to human tissue. Action Level A concentration designated in 29 CFR part 1910 for a specific substance, calculated as an eight (8)hour time-weighted average, which initiates certain required activities such as exposed monitoring and medical surveillance. Acute Toxicity Refers to adverse effect suffered as the result of a short, one-time exposure to toxic materials. It occurs within a relatively short period. Exposure is measured in seconds, minutes, or hours relative to inhalation or skin absorption. Base An organic or inorganic compound that has a pH of greater than 7. Bases are also referred to as alkalis or caustic materials and are corrosive to human tissue. Boiling Point The temperature at which the vapor pressure of a liquid is equivalent to the surrounding atmospheric pressure, and the liquid rapidly becomes a vapor. Flammable substances possessing low boiling points are considered fire hazards. Carcinogen A chemical is considered to be a carcinogen if: a. It has been evaluated by the International Agency for Research on Cancer [IARC] and found to be a carcinogen or potential carcinogen; It is listed as a carcinogen or potential carcinogen in the Annual Report on Carcinogens published by the National Toxicology Program (NTP) (latest edition); or It is regulated by OSHA as a carcinogen. Caustic Any strongly alkaline material that produces either corrosion or irritation to living tissue. Chemical Hygiene Plan A written program developed and implemented by the employer which sets forth procedures, equipment, personal protective equipment, and work practices that are capable of protecting employees from the health hazards presented by hazardous chemicals used in that particular workplace. Chemical Reactivity The ability of a material to chemically change, possibly resulting in explosion hazards or the liberation of toxic fumes. Chronic Toxicity Adverse health effects resulting from repeated or long-term exposure to toxic materials. 47 | P a g e Combustible Liquid Any liquid having a flashpoint at or above 100° F (37.8° C) but below 200° F (93.3° C), liquid except any mixture having components with flashpoints of 200o F (93.3° C), or higher, the total volume of which make up 99 percent or more of the total volume of the mixture. Compressed Gas a. A gas or mixture of gases having in a container, an absolute pressure gas exceeding gas 40 psi at 70° F (21.1° C); or b. A gas or mixture of gases having in a container, an absolute pressure exceeding 104 psi at 130° F (54.4° C) regardless of the pressure at 70° F (21.1° C); or c. A liquid having a vapor pressure exceeding 40 psi at 100° F (37.8° C) as determined by ASTM D-323-72. Corrosive A chemical that causes visible destruction of, or irreversible alterations in, living tissue by chemical action at the site of contact. Cryogenic Liquid Severely cold (-60° C to -270° C) and pressurized liquids. They present explosion hazards and can cause damage to living tissue. Designated Area An area that may be used for work with "select carcinogens," reproductive toxins, or substances which have a high degree of acute toxicity. A designated area may be the entire laboratory, an area of a laboratory or a device such as a laboratory hood. Embryotoxin A substance deemed to adversely affect a developing embryo at a particular concentration, but does not affect the pregnant female. EPA The Environmental Protection Agency federally regulates and enforces environmental protection. Explosive A chemical that causes a sudden, almost instantaneous release of pressure, gas, and heat when subjected to sudden shock, pressure, or high temperature. Flammable Liquid Any liquid having a flashpoint below 100° F (37.8° C) except any mixture having liquid components with flashpoints of 100° F (37.8° C) or higher, the total of which make up 99 percent or more of the total volume of the mixture. Flammable Solid A solid that is liable to cause a fire through friction, absorption of moisture, solid spontaneous chemical change, or retained heat from manufacturing or processing, or which can be ignited readily and when ignited burns so vigorously and persistently as to create a serious hazard. Flammability The ease with which a liquid, solid, or gas will ignite, either spontaneously (pyrophoric) or as the result of a spark or an open flame. The more flammable a material, the more readily ignition occurs. 48 | P a g e Flashpoint The minimum temperature at which a liquid gives off a vapor in sufficient concentration to ignite. Fume hood A device located in a laboratory, enclosure on five sides with a movable sash or fixed partial enclosed on the remaining side; constructed and maintained to draw air from the laboratory and to prevent or minimize the escape of air contaminants into the laboratory; and allows chemical manipulations to be conducted in the enclosure without insertion of any part of the employee's body other than hands and arms. Hazardous Chemical A chemical for which there is statistically significant evidence based on at least one study conducted in accordance with established scientific principles that acute or chronic health effects may occur in exposed employees. The term "health hazard" includes chemicals which are carcinogens, toxic or highly toxic agents, reproductive toxins, irritants, corrosives, sensitizers, hepatotoxins, nephrotoxins, neurotoxins, agents which act on the hematopoietic systems, and agents which damage the lungs, skin, eyes, or mucous membranes. Highly Toxic A chemical falling within any of the following categories: a. A chemical that has a median lethal dose (LD50) or 50 milligrams or less per kilogram of body weight when administered orally to albino rats weighing between 200 and 300 grams each. b. A chemical that has a median lethal dose (LD50) of 200 milligrams or less per kilogram of body weight when administered by continuous contact for 24 hours (or less if death occurs within 24 hours) with the bare skin of albino rabbits weighing between two and three kilograms each. c. A chemical that has a median lethal concentration (LC50) in air of 200 parts per million by volume or less of gas or vapor, or 2 milligrams per liter or less of mist, fume, or dust, when administered by continuous inhalation for one hour (or less if death occurs within one hour) to albino rats weighing between 200 and 300 grams each. Irritant Chemical substances that cause tissue inflammation or soreness upon absorption, inhalation, or ingestion. LD50 The quantity of material that when ingested, injected, or applied to the skin as a single dose, will cause death of 50% of the test animals. The test conditions should be specified, the value is expressed in g/kg or mg/kg of body weight. 49 | P a g e MSDS Material Safety Data Sheets are produced by chemical manufacturers and importers. They relay chemical, physical, and hazard information about specific chemicals. Mutagen Chemical compounds that induce mutations in DNA and living cells. Neutralize To alter an acidic or basic compound to a pH of 7, thereby making it chemically neutral. Organic Materials Any chemical compound containing carbon. Oxidizer A chemical that initiates or promotes combustion in other materials, thereby causing fire either of itself or through the release of oxygen or other gases. PEL Permissible exposure limits for the work place, set by regulation and enforced by OSHA. Most of these limit values were originally set, by consensus, by the ACGIH to assist industrial hygienists in implementing exposure control programs. As law, these are listed in 29 CFR 1910.1000 and subject to revision through the regulatory process. Poison Any substance which is harmful to living tissue when applied in small doses. Determining factors include concentration, exposure time, particle size, the substance's affinity for tissue, and sensitivity of the exposed tissue to that compound. Pyrophoric Material Any solid or liquid that has the property of spontaneous ignition in air. Radioactivity Nuclear transformation, either by natural or artificial means, resulting in emission of energy in the form of alpha, beta, or gamma rays. Amounts of radioactive material are described by the rate of radioactive decay, the Curie (Ci), or in metric multiples and fractions thereof. Reactivity The proclivity of a compound to chemically react with other substances or itself, resulting in the liberation of energy. Can cause the formation of toxic or corrosive materials, pressure buildup, and temperature fluctuations. Reproductive Toxins Chemicals which affect the reproductive capabilities including chromosomal damage (mutations) and effects on fetuses (teratogenesis). Sensitizer A chemical that causes a substantial proportion of exposed people or animals to develop an allergic reaction in normal tissue after repeated exposure to the chemical. STEL A 15-minute time-weighted average exposure which should not be exceeded at any time during a work day, even if the eight-hour time-weighted average is within the TLV. Teratogens Chemical and physical agents which interfere with normal embryonic development. Teratogens may produce congenital malformations or death of the fetus without inducing damage to the pregnant 50 | P a g e female. TLV Threshold Limit Value indicates the concentration of a chemical substance in the atmosphere that is considered non-hazardous in a person's normal working life. TWA Time Weighted Average is the concentration for a normal 8-hour working day (40 hours/week) to which all workers may be exposed without adverse effect. Toxic A chemical falling within any of the following categories: a. A chemical that has a median lethal dose (LD50) of more than 50 milligrams per kilogram but not more 500 milligrams per kilogram of body weight when administered orally to albino rats weighing between 200 and 300 grams each. b. A chemical that has a median lethal dose (LD50) of more than 200 milligrams per kilogram but not more than 1000 milligrams per kilogram of body weight when administered by continuous contact for 24 hours (or less if death occurs within 24 hours) with the bare skin of albino rabbits weighing between two and three kilograms each. c. A chemical that has a median lethal concentration (LC50) in air of more than 200 parts per million but not more than 2,000 parts per million by volume of gas or vapor, or more than two milligrams per liter but not more than 20 milligrams per liter of mist, fume, or dust, when administered by continuous inhalation for one hour (or less if death occurs within one hour) to albino rats weighing between 200 and 300 grams each. Ultraviolet Light Radiation in the electromagnetic spectrum (wavelengths of 100 - 3900 Angstroms). Volatility The tendency of a liquid or solid to pass into the vapor state at a particular temperature. Water Reactive A chemical that reacts with water to release a gas that is either flammable or presents a health hazard. 51 | P a g e REFERENCES American Chemical Society. Safety in Academic Chemistry Laboratories, Mar. 1974. Bretherick, L. eds. Hazards in the Chemical Laboratory, London: Royal Society, 1986. Bretherick, L. Handbook of Reactive Chemical Hazards, London: Butterworths, 1981. Campus Safety Office. Laboratory Safety Manual; U.N.C.A. University of North Carolina, Ashville. Committee on Hazardous substances in the Laboratory Assembly of Mathematical and Physical Sciences; National Research Council. Prudent Practices for Handling Chemicals. Washington D.C. National Academy Press, 1981. Office of Environmental Health and Safety University of Virginia Special Material Handling Facility. Chemical Safety Guide Charlottesville: 1988. Department of Chemistry Indiana University. Laboratory Safety Manual. Bloomington: Aug. 1989. Department of Risk and Safety. University of Arizona Safety Manual. Division of Occupational Health Task Force on Hazard Communication Virginia Department of Labor and Industry. Hazard Communication Standard (1910.1200) An Information Manual. Richmond: Sept. 1985. Freeman, N.T. and J. Whitehead. Introduction to Safety in the Chemical Laboratory. Orlando. Academic Press Inc., 1982. Occupational Health and Safety Division. Argone National Laboratory Health and Safety Manual 1 May 1973. Right-to-Know Pocket Guide for School and University Employees. Genium Publishing Corporation, chenectady, NY 1990. Singer, James; Pesticide Safety: Guidelines for Personnel Protection; Davis, October, 1982. Supervisors Safety Manual. 6th ed. Chicago: National Safety Council, 1985. U.S. Department of Health, Education, and Welfare et al.; Hazards in the Chemistry Laboratory; Student Manual Sept. 1979. U.S. Department of Health, Education, and Welfare, et al.; NIOSH Health and Safety Guide for Pesticide Formulators; Cincinnati, May, 1977. U.S. Environmental Protection Agency, Private Pesticide Applicators: Training Manual, February, 1989. Virginia Polytechnic Institute and State University Safety and Health Program. Laboratory Safety Manual 1 Jan. 1983. 52 | P a g e