lecture notes
advertisement

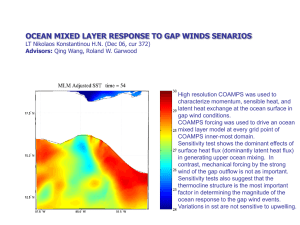
Chem 1103: Lecture 4: Properties of Seawater and Ocean Circulation (Deep Circulation) : finish of Chap 5 & 6 Gases in Seawater o The solubility and saturation value for gases in sea water increase as temperature and salinity decrease and as pressure increases Solubility is the ability of something to be dissolved and go into solution Solubility of gases measured in ml gas/ l for this book/course Saturation value is the equilibrium amount of gas dissolved in water at an existing temperature, salinity, and pressure Water is undersaturated when under existing conditions it has the capacity to dissolve more gas. Gas content is below the saturation value Water is saturated when under existing conditions it contains as much dissolved gas as it can hold in equilibrium. Gas content is at the saturation value. Water is supersaturated when under existing conditions it contains more dissolved gas than it can hold in equilibrium. Gas content is above saturation value and excess gas will come out of solution Sources of oxygen and carbon dioxide Atmosphere Volcanoes underwater Biology – respiration, photosynthesis, decomposition o Respiration - O2 in CO2 out o Photosynthesis – CO2 in O2 out o Decomposition -CO2 in O2 out Sinks of oxygen and carbon dioxide Biology – respiration, photosynthesis, decomposition The surface layer is usually saturated in atmospheric gases because of direct exchange with the atmosphere Below the surface layer, gas content reflects relative importance of respiration, photosynthesis, decay, and gases released from volcanic vents. o Oxygen Tends to be abundant in the surface layer and deep layer, but lowest in the pycnocline Surface layer is rich in oxygen because of photosynthesis and contact with the atmosphere Oxygen minimum layer occurs at about 150 to 1500 m below the surface and coincides with the pycnocline Sinking food particles settle into this layer and become suspended in place because of the greater density of the water below 1 The food draws large numbers of organisms which respire, consuming oxygen Decay of uneaten material consumes additional oxygen Density difference prevents mixing downward of oxygen-rich water from the surface or upward from the deep layer The deep layer is rich in oxygen because its water is derived from the cold surface waters, which sank (convected) to the bottom. Consumption is low because there are fewer organisms and less decay consuming oxygen Anoxic waters contain no oxygen and are inhabited by anerobic organisms (bacteria) o Carbon dioxide Major sources of carbon dioxide are respiration and decay Major sinks are photosynthesis and construction of carbonate shells Carbon dioxide controls the acidity of sea water A solution is acid if it has excess H+ (hydrogen) ions and is a base if it has an excess of OH- (hydroxyl) ions pH measures how acidic or basic water is o pH of 0 to 7 is acidic o pH of 7 is neutral o pH of 7 to 14 is basic seawater has a pH of 7.8 to 8.2 pH is related to the amount of CO2 dissolved in water because it combines with the water to produce carbonic acid which releases H+ ions CO2 +H2O H2CO3H+ + HCO3- 2H+ +CO3-2 H2CO3 is carbonic acid, HCO3- is the bicarbonate ion, and CO32 is the carbonate ion Changing the amount of CO2 shifts the reaction to either the right or left of the equation o Adding CO2 shifts the reaction to the right and produces more H+ ions making the water more acid o Removing CO2 shifts the reaction to the left, combining H+ ions with carbonate and bicarbonate ions reducing the acidity Dissolved CO2 in water acts as a buffer, a substance that prevents large shifts in pH Dissolution of carbonate shells in deep water results because cold water under great pressure has a high saturation value for CO2 and the additional CO2 releases more H+ ions making the water acid Warm, shallow water is under low pressure, contains less dissolved CO2 and is less acidic. Carbonate sediments are stable and do not dissolve The Ocean as a Physical System o Water is recycles from the ocean to the land and returned to the sea 2 The reservoirs of water include Oceans – cover 60% of the northern hemisphere and 80% of the southern hemisphere and contain 97% of Earth’s water Rivers, lakes and glaciers Groundwater – contains a larger volume of water than all of the water in the lakes and rivers The hydrologic cycle describes the exchange of water between ocean, land and atmosphere Table of reservoirs On land precipitation exceeds evaporation In the ocean evaporation exceeds precipitation The ocean is part of a biogeochemical system in which land undergoes weathering and weathered products are transported to the sea where they may be deposited directly or used by organisms and later deposited as organic remains or organic wastes. Deposits are buried, lithified, and recycled by plate tectonics into new land which is weathered an the cycle repeats Other Physical Properties of Water o Light Amount of light entering the ocean depends upon the height of the sun above the horizon and the smoothness of the sea surface 65% of light entering the ocean is absorbed within the first meter and converted into heat. Only 1% of light entering the ocean reaches 100m Water displays the selective absorption of light with long wavelengths absorbed first and short wavelengths absorbed last In the open ocean, blue light penetrates the deepest In turbid coastal waters, light rarely penetrates deeper than 20 m and the water appears yellow to green because particles reflect these wavelengths The photic zone is the part of the water column penetrated by sunlight The aphotic zone is the part of the water column below light penetration and permanently dark o Speed of sound The speed of sound in water increases as salinity, temperature and pressure increase, but in the ocean is mainly a function of temperature and pressure Above the pycnocline, increasing pressure with depth increases the speed of sound despite the gradual decrease in temperature Within the pycnocline, the speed of sound decreases rapidly because of he rapid decrease in temperature and only slight increase in pressure Below the pycncline, the speed of sound gradually increases because pressure continues to increase, but temperature only declines slightly SOFAR channel is locate where sound speed is at a minimum. Refraction of sound waves within the channel prevents dispersion of the sound energy and sound waves travel for thousands of km within the channel 3 Deep Ocean Circulation o Define deep water o Density driven, not wind driven - convection o Thermohaline circulation is density driven flow of water generated by differences in salinity of temperature Water at the surface is exposed to more rapid changes in salinity through evaporation or precipitation and in temperature through cooling or heating Once water is isolated from the atmospheric influences, salinity and temperature are largely set for an extended period of time Based upon depth, surface water masses can be broadly classified as: Central waters – from 0 to 1 km Intermediate waters - from 1 to 2 km Deep and bottom waters – greater than 2 km Most deep and bottom water originated at the surface where cooling and increased salinity raised their density until they sank Ocean basins interconnect and exchange water with each other and with the surface. Inter-ocean basin circulation and exchange between surface and deep water appears largely driven by waters of he North Atlantic o The major thermohaline currents appear to flow mainly equatorward, but this is because they originate in the polar regions and their outward flow is confined between the continents Warmer water (>10oC) is confined between 45o N and S latitude Poleward of 45o, density of water increases because of declining temperature and increased salinity because of evaporation or ice formation The water sinks to a density-appropriate level and then slowly flow outward in all directions across the basin until they are blocked by a continent Deep water gradually mixes with other water masses and eventually rises to the surface Do a demo o Explain the Conveyer Belt o The Atlantic Ocean has the most complex ocean stratification, containing the following layers: Antarctic Bottom Water forms in the Weddell Sea mainly in the winter when water is very cold and ice formation increase salinity It is the densest water in the ocean and flows northward into the North Atlantic Antarctic Circumpolar Water forms a less saline and slightly warmer water mass off the coast of Antarctic where conditions are not as severe- Note book calls this Antarctic Deep Water It flows northward at the surface to the Antarctic convergence, where it sinks and flows southward along the bottom until it encounters and rides over the denser Antarctic Bottom Water 4 North Atlantic Deep water forms a cold, salty water mass off the coast of Greenland that sinks and flows southward along the bottom until it encounters and rides over the denser Antarctic Bottom Water Arctic Intermediate Water forms in the Subarctic region and flows southward between the surface water of the Sargasso Se and the North Atlantic Deep Water Mediterranean Intermediate Water flows out of the Mediterranean Sea as a warm, highly saline water of the Sargasso Sea and the North Atlantic Deep Water The Pacific Ocean has a less complex stratification, is weakly layered, displays sluggish circulation and is remarkably uniform below 2000m Common Water is the main water mass and is formed by a blending of the Antarctic Bottom Water and North Atlantic Deep Water in the Antarctic Circumpolar Current o It is injected into the Pacific basin and flows northward to 10oS where it meets Pacific Subarctic Water Above 2000m, the North Pacific Intermediate water and Antarctic Intermediate water dominate The Indian Ocean has the simplest stratification consisting of : Common water which occupies most of the basin Antarctic Intermediate water Red Sea intermediate water o It is a warm and highly saline (40 psu) water from the Red Sea that flows into the Indian Ocean and sinks to about 3000 m Water flow in semi-enclosed seaways o Most seas are indentations into continents, partially isolated from the ocean and strongly influenced by continental climate and river drainage As Atlantic Ocean water flows through the Straits of Gibraltar into the Mediterranean Sea at the surface, warm, highly saline Mediterranean Sea water flows out through the Straits at the bottom As the surface water flows eastward, salinity increases because of excess evaporation Sea surface slopes eastward and surface water flows down the resulting pressure gradient At the eastern edge of the basin the warm but highly saline (38 psu) water sinks, flows westward and eventually out into the Atlantic Ocean as Mediterranean Intermediate Water where it sinks to 1000 m Mediterranean Sea is nutrient poor because there is no upwelling of deep water to resupply nutrients German subs Lab 1. Explain temperature, density, plotting profiles 5 2. Explain water masses Bring 1. Long tank 2. sugar water 3. sugar 4. big spoon 5. food color 6. float objects 7. soda water 8. soda 9. pH paper 10. sugar, salt, water and containers 11. 6