Chemistry-Mathematics - ILAPs Project at TU
advertisement

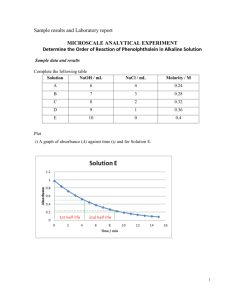
Chemistry-Mathematics ILAP Chemical Kinetics Fading of Phenolphthalein Authors William Potter Shirley Pomeranz Keith Symcox 1 Interdisciplinary Lively Applications Project (ILAP) Title: Chemical Kinetics: The Rate of a Reaction Fading of Phenolphthalein Authors: William Potter, Department of Chemistry and Biochemistry Shirley Pomeranz, Department of Mathematical and Computer Sciences Keith Symcox, Department of Chemistry and Biochemistry The University of Tulsa, Tulsa, OK Mathematics Classifications: Calculus I - MATH 2014 (Derivatives; Rate of change) Disciplinary Classification: General Chemistry II – CHEM 1023 (Chemical kinetics; Rate of reaction) Prerequisite Skills: 1. Graphing and analyzing graphs 2. Differentiation Physical Concepts Examined: 1. Chemical kinetics: Rate of reactions Materials Available: 1. Problem statement and discussion; Student 2. Supplemental background material; Student 3. Sample solution; Instructor 4. Notes for Instructors 5. References; Student Computing Requirements: Basic graphing capability using Excel, Mathematica, MATLAB, or another graphing package/tool Class Requirements: One class period for laboratory experiment (requires spectrophotometer, burette and standard solutions; One class period for problem plots Partial support for this work was provided by the National Science Foundation's Course, Curriculum, and Laboratory Improvement program under grant #0410653 2 Problem statement and discussion – Chemistry experiment: Introduction: Your calculus text gives many examples of determining rates of change, and we now investigate how this same mathematical background is used to evaluate chemical reaction rates. “Chemical Kinetics” is the branch of chemistry that measures the rate of a reaction- the speed at which one chemical reacts to form another. The relationship between a reaction's rate and the concentration of a chemical species that can affect the rate is given by a "rate law." The rate takes the general form: rate = k [A]B[E] where k is the "rate constant," and A, B, and E are chemical species whose concentrations affect how quickly the reaction occurs. In chemical shorthand notation, the concentration of the chemical is put within square brackets, e.g. [A], and will typically have units of moles/liter or Molarity (abbreviated M). The superscripts associated with each concentration term (e.g. andare called "reaction orders." The reaction orders may be positive or negative integers; they may even be zero or fractional. The information provided by the relation between "reaction rate" and a particular compound's "reaction order" helps chemists to understand the specific way or "mechanism" in which a reaction occurs. (1) In this ILAP you will do an experiment using common chemicals to determine reaction rates. You will observe a reaction between the active ingredient in Ex-lax® (phenolphthalein- a pinkish/ red molecule under "basic" -or "alkaline"- conditions) and the active ingredient in Drâno® (hydroxide, OH-)- a strong base. Phenolphthalein is commonly used as an "indicator dye" to follow changes in pH. In this experiment, however, you will be monitoring the final "fading of phenolphthalein" to a colorless compound that occurs upon reaction with a strong base. pH < 7 pH 8-10 Final product at high pH Figure 1 The rate at which a reaction depends on concentration is called "reaction order" or “order of the chemical reaction” (see equation 1 above). The phrase “first order” is chemistry terminology used to mean that the rate at which the reaction is taking place is directly proportional to the concentration of that reactant. For example, in our example, if the reaction is first order with 3 respect to [A], then if the concentration of A is doubled, then the reaction should occur twice as fast as it originally occurred. If three times the amount of A is used, then the reaction rate is three times as fast. In a "first order" reaction the exponent (for [A]) is one. The form of the rate equation is (2) rate = k [A] [B] ….[E] Note: Since the exponent for A is one, it is not written in the equation. Be careful not to confuse chemistry and mathematics terminology. The same terminology in mathematics can mean something different. In mathematics, the term “order”, when used in the description of differential equations, refers to the highest order derivative that appears in the differential equation. Thus, for example, if d2y is the highest order derivative that appears, then the dx 2 differential equation is said to be of second order. Procedures: Safety Precautions. Use safety goggles when performing this experiment. Hydroxide is a strong caustic agent and should be handled with care. Do not let it touch eyes, skin or clothes. If sodium hydroxide is spilled, clean the area with plenty of water. If you feel a slippery feeling, you are touching the strong base, and the fats in your fingers are hydrolyzing! Disposal. All the solutions should be poured down the sink with extra water. Setting up the spectrophotometer to measure the fading of phenolphthalein. (A spectrophotometer can measure absorbance, how much light is absorbed.) Note: A demonstration run will be done by your instructor. 1. Turn on the spectrophotometer and make sure the wavelength is set to 550 nm. 2. Set the Absorbance value to 0.00 using the 0.3 M NaCl "blank" test tube provided. 3. Place 5.00 ml of the following NaOH (sodium hydroxide) solutions into four separate test tubes. Table I Trial Concentration NaOH (M) 1 0.24 2 0.32 3 0.40 4 0.48 4. Make sure you have a stopwatch or a watch with a second hand. Start the watch as you add 3 drops of phenolphthalein solution into Trial #1. Mix quickly and thoroughly without splashing. Place the tube into the spectrophotometer and read and record the absorbance data on the data sheet below every 15 seconds (i.e. 0.25 minute) until 5 minutes have passed. 5. Repeat step 4 for each of the three other NaOH solutions. Repeat any trials as needed. Disposal. All the solutions can be poured safely down the sink with extra water. 4 Data Sheet Time (minutes) Trial 1 Trial 2 Trial 3 Trial 4 [NaOH]= 0.24M Absorbance [NaOH]= 0.32M Absorbance [NaOH]= 0.40M Absorbance [NaOH]= 0.48M Absorbance 0 0.25 0.50 0.75 1.00 1.25 1.50 1.75 2.00 2.25 2.50 2.75 3.00 3.25 3.50 3.75 4.00 4.25 4.50 4.75 5.00 Data Workup and Chemical Assignment: 1. Make two graphs. Graph (1) will be absorbance versus time and graph (2) will be the natural log of the absorbance (ln A) versus the time in minutes for each trial. As you have done four runs, there will be four lines on each graph. The graphs should be page sized, properly titled, with the equation for each line printed on the graph. The magnitude (absolute value) of the slope of line in the ln A versus time plot is the pseudo-rate constant (k' ) for that trial. (This is explained further below.) Note that k' is a symbol denoting a (positive) number, not a derivative. 2. Graph the natural log of the pseudo-rate constant, ln(k' ), versus the natural log of the particular OH- concentration (in M). Find the slope, y intercept and equation for the line. 3. Find the rate constant k. The rate constant, k, is found from the y intercept of the graph of ln (k' ) versus ln [OH-] (note: ln [OH-] = ln [NaOH-]) . The y intercept is ln k, so k = e ln k. (See discussion below.) 4. Find the reaction order with respect to hydroxide [OH-]. From the discussion given below, you can see that the reaction order with respect to [OH-] is equal to the slope of the line from the data workup step 2. 5 What You Did and Why: This reaction is a simple example of the relationship between concentration and rate, and how to calculate a rate law for a chemical reaction. As you should see from your data, the rate of reaction is increased as the concentration of NaOH goes up. The reaction of phenolphthalein (abbreviated P) with hydroxide (OH-) to give a colorless compound (POH) can be written as: (3) P + OH- → POH The rate law for this reaction is (4) rate = - k [OH-] [P] where k is a positive number. The negative sign for the rate constant is used because the reactants are being used up. The exponent terms, or , correspond to the reaction order. As we did the experiment, the hydroxide concentration, [OH-], is vastly greater than the Phenolphthalein concentration, [P]. Thus, we can consider that the [OH-] stays almost constant. This means that we could come up with a new rate constant (k') (called a "pseudo-rate constant") that includes the original rate constant, k, and the [OH-]: (5) k' = k [OH-] Substituting equation (5) into equation (4), simplifies the rate law to (6) rate = - k' [P ] where k' is called the pseudo-rate constant. Graphs 1 & 2. The absorbance (amount of light absorbed) of the phenolphthalein is proportional to its concentration (this is known in chemistry as "Beer's law" i.e., Absorbance = ε × M × l, where ε is a positive constant of proportionality, M is concentration, and l is the sample pathlength). As you will discover in your mathematics analysis section below, in a first order kinetic reaction, a graph of the natural log of the concentration versus time will be a straight line, and the magnitude of the slope of this line will equal the rate constant. Therefore, for your graphs, the natural log (Absorbance) versus time should be linear, since the reaction is expected to be first order with respect to [P], i.e., = 1. The slope of each line will be equal to k', the pseudo-rate constant for the given concentration of OH-. 6 Graph 3. The third graph is a graph of ln (k') versus ln [OH-]. Since, from equation (5), we see that, taking the natural log of both sides, we can derive ln (k') = ln ln k (6.5) which has the form of a line y = mx +b. This means that graphing ln (k') versus ln [OH-] will give us a line with a slope equal to the order of the reaction for hydroxide ( and a y intercept equal to the natural log of the rate constant (ln k). The rate constant can then be calculated and reported in your data along with the reaction order for hydroxide. The Mathematical View: At the concentrations that were used for the experiments, absorbance of the phenolphthalein is proportional to the concentration of the phenolphthalein, and therefore, to the amount of phenolphthalein present. Therefore, it turns out that we can interpret the following equations, Eq (7) and Eq (8), using absorbance and concentration in the sense described below. The mathematics notation for rate that corresponds to the chemistry notation is (7) d y (t ) k ( z (t )) y (t ) , dt where k is the positive constant of proportionality (rate constant); y is the absorbance of phenolphthalein as a function of time, t; z is the concentration of sodium hydroxide as a function of time, t; α is the reaction order with respect to sodium hydroxide; As in the experiments, we can assume that the amount of sodium hydroxide is in large excess compared to the amount of phenolphthalein. This implies that z(t), the concentration of sodium hydroxide, is approximately constant. For mathematical modeling purposes, we can then rewrite Eq (7) as (8) d y (t ) kˆ y (t ) , dt Where k̂ is called the pseudo-order rate constant, k̂ = k z(t)α, and where z has been assumed constant. Note: In the mathematical formulation, we choose to use k̂ , whereas in the chemical formulation, we used k'. In chemistry, the primes often denote a pseudo-rate constant, whereas in mathematics, k' often denotes the first derivative. 7 Mathematical Assignment: Part (1). You are to solve the general Eq (8) analytically for y(t), the absorbance of phenolphthalein as a function of time, (separable ordinary differential equation) or, from your familiarity with derivatives (make a clever guess for the solution, and then check your guess), subject to the initial condition, y(0) = y0 , where y0 is a constant. The parameters y0 and k̂ will appear in your solution. Part (2). Determine the four solutions for y(t), one solution corresponding to each of the four concentrations of NaOH. Use your result from Part (1) here, and just substitute the numbers for y0, the initial absorbance of the phenolphthalein, and k̂ , the pseudo-rate constant. The four solutions will be different, but will have the same general form. The only way in which they differ is in the values for k̂ (i.e., k' ), obtained from the experiments. You will obtain numbers for the values of y0 and k' from the experiment, and use these numbers in your formula for y. Part (3). Do the following for each of the four concentrations of NaOH. You will have four separate plots, each with a continuous function determined analytically (mathematically) and its corresponding discrete data points (obtained experimentally): Plot y as a function of time, t, for both your continuous (analytical) solution (from Part (2) here) and your numerical data from your experiment (which will consist of discrete data points). Plot these results on the same set of axes, clearly labeled, etc. Observe if your results are consistent. If they are not, discuss some possible sources of error. Clearly include your group's observations and conclusions in your report. Explain any assumptions that have been made in the mathematical modeling of the problem which may account for discrepancies between what you have obtained analytically versus what you observed experimentally. You can refer to sections of the text, credible websites, or other sources. Cite all sources, as appropriate. In particular, acknowledge any individuals who assist you. However, for each group, your work must be your own. Submit your report with all graphs (clearly labeled) and supporting material (professionally presented). Use the notation provided in this discussion. Use the guidelines that you have been given for writing a science/engineering/mathematics report. (See “Technical Report Format and Writing Guide” at the TU ILAPs web site: http://www.ilaps.utulsa.edu/). You can use your calculus book to get examples of professional writing styles for mathematics and text (your instructors may have additional handouts or references). Supplemental background material: The following site is very, very helpful for understanding the phenolphthalein kinetics. http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch22/rate.html The following mathematics modules can help you find supplementary information about separable ordinary differential equations (you may find other web sites with useful information about ordinary differential equations that describe exponential decay): http://www.math.eku.edu/jones/sect1_5.pdf 8 http://www.physics.uoguelph.ca/tutorials/exp/decay.html http://www.math.wpi.edu/Course_Materials/Calc3/Labs/node2.html The following sites have general information about chemical kinetics. http://dept.physics.upenn.edu/courses/gladney/mathphys/subsection2_1_4.html http://chem.csustan.edu/perona/1112/1112Kinetics/ http://metric.ma.ic.ac.uk/~phil/chem/index.html (see Lab 6: Reaction kinetics and differential equations) Table 2 is taken from this last website. Order 0 1 2 n Rate law Integrated rate law da = - k dt da = - ka dt d a = - k a2 dt k t = a0 - a 3 d a = - k a3 dt ³ 2 d a = - k an dt JN J N a0 a k t = ln 1 kt= kt= kt= 1 n- 1 - a 1 - 2 a2 1 an - 1 1 a0 1 2 a02 - 1 a0n - 1 Table 2 -------------------------------------------------------------------------------------------------------------------- References: [1] Gabriela C. Weaver, Doris R. Kimbrough; Overhead demonstrations, Journal of Chemical Education, 1996, 73, 256, http://www.jce.divched.org/cgi-bin/JCE/jce-idx.pl?type=goto&volume=73&issue=3&page=256 [2] Larry Gladney, University of Pennsylvania, Department of Physics and Astronomy, http://dept.physics.upenn.edu/courses/gladney/mathphys/subsection2_1_4.html [3] Michael Perona, California State University – Stanislaus, Chemistry Department, http://chem.csustan.edu/perona/1112/1112Kinetics/ [4] Phillip Kent, Philip Ramsden, David Klug, Richard Templer; Imperial College, The METRIC Project, Department of Mathematics, The Chemistry Maths Lab, Lab 6: Reaction kinetics and differential equations, full text of the notes for this lab: Mathslab6.nb, http://metric.ma.ic.ac.uk/~phil/chem/index.html also see http://library.wolfram.com/infocenter/Courseware/3767/ select: Science > Chemistry [5] Josefina Arce, Rosa Betancourt-Perez, Rivera Yamil, Joan Pijem; The reaction of a food colorant with sodium hypochlorite, Journal of Chemical Education, 1998, 75, 1142, http://www.jce.divched.org/Journal/Issues/1998/Sep/PlusSub/V75N09/p1142.pdf 9