Sample Report
advertisement

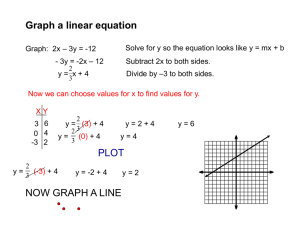
Sample results and Laboratory report MICROSCALE ANALYTICAL EXPERIMENT Determine the Order of Reaction of Phenolphthalein in Alkaline Solution Sample data and results Complete the following table Solution NaOH / mL NaCl / mL Molarity / M A 6 4 0.24 B 7 3 0.28 C 8 2 0.32 D 9 1 0.36 E 10 0 0.4 Plot i) A graph of absorbance (A) against time (t) and for Solution E. 1 ii) A graph of ln absorbance (ln A) against time (t) for Solution A – E Slope = – 0.1694, R2= 0.999 Slope = – 0.2413, R2= 0.999 Slope = – 0.2047, R2= 0.999 Slope = – 0.269, R2= 0.999 Slope = – 0.3216, R2= 0.999 2 Questions 1. From the plot of A vs t, determine the half life (t1/2) of the reaction for each NaOH concentration. Half life of Solution E (0.4M) is about 4.4 minutes 2. From the plot of ln A vs t, determine a) the reaction order with respect to phenolphthalein. The plot of ln A versus time gives an excellent straight line for any given NaOH concentration, and the half life of the reaction is constant, so the reaction is first order with respect to phenolphthalein. b) the rate constant, k1, for each NaOH concentration. The rate constant k1 is the slope of each graph so, k1 for Solution A is 0.1694 min-1, k1 for Solution B is 0.2047 min-1 k1 for Solution C is 0.2413 min-1, k1 for Solution D is 0.269 min-1 k1 for Solution E is 0.3216 min-1 3. Using the result in (2b), plot a graph of ln k1 against ln [NaOH]. Slope = 1.2, R2 = 0.995 3 a) From the graph, determine the reaction order with respect to NaOH. Since the slope of the plot of ln k1 against ln [NaOH] equals 1.2, so the reaction is first order with respect to NaOH. b) What is the total reaction order? The reaction is first order with respect to phenolphthalein and first order with respect to NaOH, so the total reaction order is 2. 4