Homework #3
advertisement
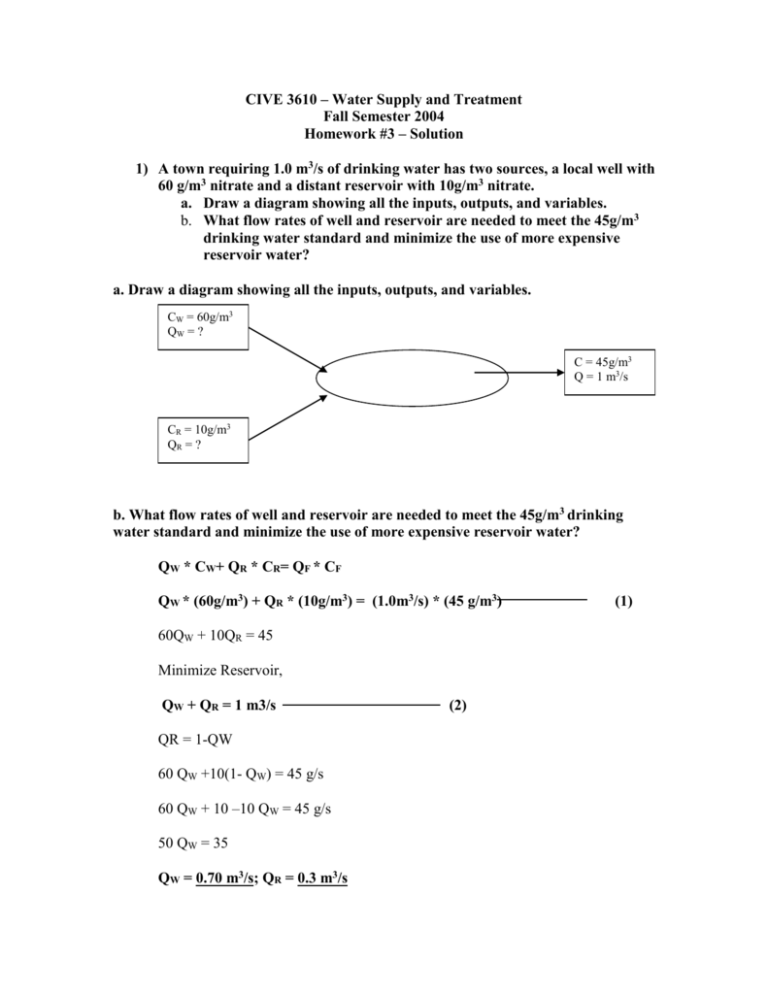
CIVE 3610 – Water Supply and Treatment Fall Semester 2004 Homework #3 – Solution 1) A town requiring 1.0 m3/s of drinking water has two sources, a local well with 60 g/m3 nitrate and a distant reservoir with 10g/m3 nitrate. a. Draw a diagram showing all the inputs, outputs, and variables. b. What flow rates of well and reservoir are needed to meet the 45g/m3 drinking water standard and minimize the use of more expensive reservoir water? a. Draw a diagram showing all the inputs, outputs, and variables. CW = 60g/m3 QW = ? C = 45g/m3 Q = 1 m3/s CR = 10g/m3 QR = ? b. What flow rates of well and reservoir are needed to meet the 45g/m3 drinking water standard and minimize the use of more expensive reservoir water? QW * CW+ QR * CR= QF * CF QW * (60g/m3) + QR * (10g/m3) = (1.0m3/s) * (45 g/m3) 60QW + 10QR = 45 Minimize Reservoir, QW + QR = 1 m3/s QR = 1-QW 60 QW +10(1- QW) = 45 g/s 60 QW + 10 –10 QW = 45 g/s 50 QW = 35 QW = 0.70 m3/s; QR = 0.3 m3/s (2) (1) 2) During a chemical reaction, the following concentrations of Species A at various times were observed. Determine: a. The reaction order and reaction rate constant, k b. The concentration of A after 20hrs. Time, hours 0 7.5 15 22.5 30 Concentration, mg/L 50.8 32 19.7 12.3 7.6 Solution: a. The reaction order and reaction rate constant, k Assume a first order reaction. The plot of ln (Ct) versus time is shown in Figure 1. Since the plot is a straight line, the reaction is a first order reaction. The slope of line gives the rate constant ‘k’. Figure 1. Plot of ln (Ct) vs. Time ln (Ct) ln (Ct) vs. Time 4.5 4 3.5 3 2.5 2 1.5 1 0.5 0 0 7.5 15 22.5 30 Time Rate constant K = [ln (50.8) –ln (7.6)] /30hrs] = [(3.92-2.03) / 30] = 0.063/hr b. The concentration of A after 20hrs Ct/ CO = e - (k * t) Ct = (50.8 mg/L) e - (0.063 * 20) =14.3 mg/L 3) A completely mixed chemical reactor has an influent flow with a concentration of 150 mg/L of A and flowrate of 100 gal/ min. The reaction is first order and the rate constant is 0.4/hr. Determine: a. Draw a diagram showing all inputs, outputs, and variables. b. The required detention time and the volume (gallons) of the reactor if the effluent contains 20 mg/L of A. c. Draw a diagram for a plug flow reactor with the same design conditions. d. How many times larger a completely mixed reactor must be than a plug flow reactor for 80% removal or conversion. e. How many times larger a completely mixed reactor must be than a plug flow reactor for 90% removal or conversion. f. Why does a plug flow reactor perform better than a completely mixed reactor? a. Draw a diagram showing all inputs, outputs, and variables. 150mg/L CF CF 100gal/min 100 gal/min K=0.4/hr b. The required detention time and the volume (gallons) of the reactor if the effluent contains 20 mg/L of A. θ = (CO- CF) / KCF = (150-20) / (0.40*20) = 16.3 hrs Volume V= Q* θ = (100gal/min)* (60 min/hr) *16.3 hr = 97800 gals c. Draw a diagram for a plug flow reactor with the same design conditions. 150mg/L CF K=0.4/hr 100 gal/min 100 gal/min d. How many times larger a completely mixed reactor must be than a plug flow reactor for 80% removal or conversion. 80% removal = (CO- CO * 80/100) = (150 -150* (80/100)) = 150-120 = 30 Therefore, CF = 30mg/L PFR CF/CO = e– (K * θ) 30/150 = e– (0.4 * θ) θ = 4.02 hrs CSTR θ = (CO - CF)/ (K * CF ) = (150-30) / (0.4 * 30) θ = 10 hrs The ratio of CSTR to PFR CSTR / PFR = 10 / 4 = 2.5 Therefore, CSTR = 2.5 times larger than PFR e. How many times larger a completely mixed reactor must be than a plug flow reactor for 90% removal or conversion. 90% removal = (CO- CO * 90/100) = (150 -150* (90/100)) = 150-135 = 15 Therefore, CF = 15 mg/L PFR CF/CO = e– (K * θ) 15 /150 = e– (0.4 * θ) θ = 5.76 hrs CSTR θ = (CO - CF)/ (K*CF ) = (150-15) / (0.4*15) θ = 22.5 hrs The ratio of CSTR to PFR CSTR / PFR = 22.5 / 5.76 = 3.91 Therefore, CSTR = 3.91 times larger than PFR f. Why does a plug flow reactor perform better than a completely mixed reactor? Plug flow reactors (PFRs) work better than completely mixed reactors (CSTRs) because chemical conversion rates are generally first order. First order rates are directly proportional to contaminant concentration. Since it is assumed there is no dispersion in an idealized plug flow reactor, the average contaminant concentration is greater than that in a CSTR, thus the reaction rate is faster, less detention time is needed and the volume of the reactor can be smaller. 4) Three CSTRs are to be used in series. The second reactor has a volume twice that of the first and third reactors. The influent flow has a concentration of 150 mg/L of A, and the flowrate is 100 gal/min (380 l/min). The reaction is first order, the rate constant is 0.4/hr. a. Draw a diagram showing all inputs, outputs, and variables. b. Determine the mean residence time and volume of each reactor if the removal or conversion of A is 90%. c. What is the concentration of A in each reactor? d. What is the rate of conversion in each reactor? a. Draw a diagram showing all inputs, outputs, and variables. CF 150 mg/L 15 mg/L Q = 100gpm V1=V V V3=V V1=V V1=V V1=V V2=2V K=0.4/h r 100gpm K=0.4/h r b. Determine the mean residence time and volume of each reactor if the removal or conversion of A is 90%. CF = CO / [(1+Kθ1)* (1+Kθ2)* (1+Kθ3)] 15 = 150/ [(1+0.4θ) *(1+0.4*2θ) *(1+0.4*θ)] Rearrange equation, 1+1.6θ + 0.8θ2 +0.13θ3 = 10 V1= V3=V V2 = 2V We have, V= Q*θ θ = V/Q θ1= V1/Q = V/Q = θ θ2= V2/Q = 2V/Q = 2θ θ3= V3/Q = V/Q = θ Trial and error solution to get the Mean residence time θ1 = θ3 = 2.25 hrs θ2 =2 θ =2*2.25 = 4.5 hrs θ 2 3 2.5 2.25 2.20 Volume of the reactors V1= V3 = V = Qθ = (100gal/min) * (60 min/hr) * 2.25 hr =13500 gal V2 = 2V = Qθ2 = (100gal/min) * (60 min/hr) * 4.5 hr = 27000 gal c. What is the concentration of A in each reactor? C1 = [CO/ (1+Kθ1)] = 150/ (1+0.4*(2.25)) = 79 mg/L CO/CF =10 8.44 16.5 12.03 10.13 9.78 C2 = [C1/ (1+Kθ2)] = 79/ (1+0.4*(4.5)) = 28 mg/L C3 = [C2/ (1+Kθ3)] = 28/ (1+0.4*(2.25)) = 15 mg/L d. What is the rate of conversion in each reactor? r1 = [(CO – C1) / θ1] = [(150-79) / 2.25] = 32 mg/L-h r2= [(C1 – C2) / θ2] = [(79-28) / 4.5] = 32 mg/L-h r3 = [(C2 – C3) / θ3] = [(28-15) / 2.25] = 5.8 mg/L-h