6 - Human Impact on the Environment - Air Pollution
advertisement
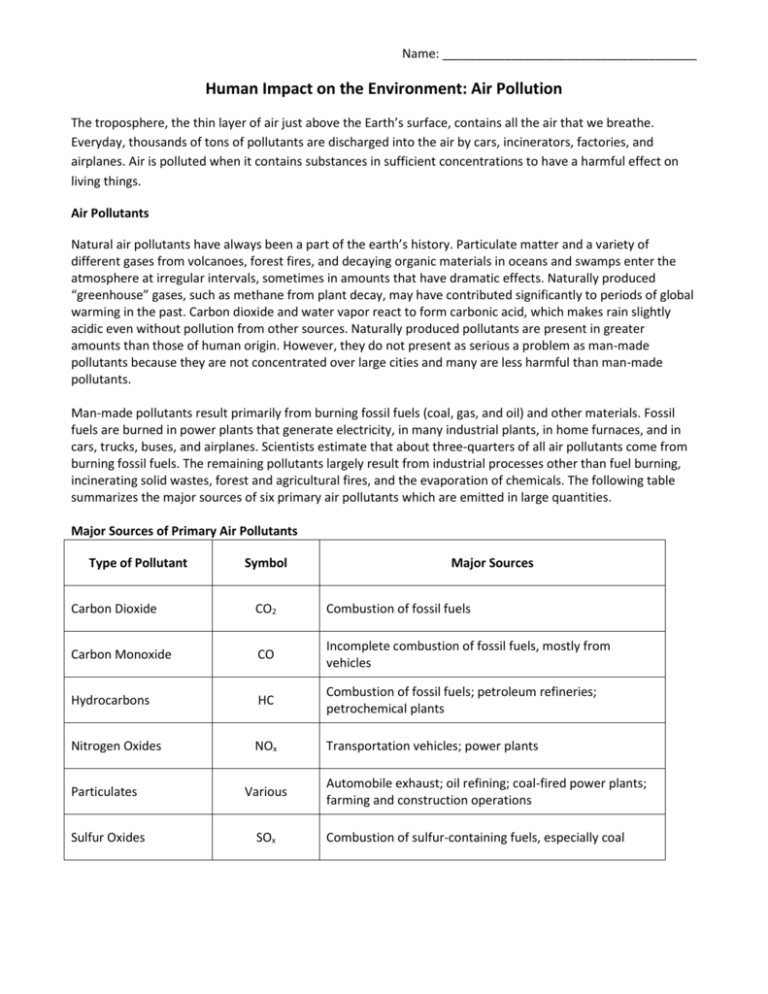
Name: _____________________________________ Human Impact on the Environment: Air Pollution The troposphere, the thin layer of air just above the Earth’s surface, contains all the air that we breathe. Everyday, thousands of tons of pollutants are discharged into the air by cars, incinerators, factories, and airplanes. Air is polluted when it contains substances in sufficient concentrations to have a harmful effect on living things. Air Pollutants Natural air pollutants have always been a part of the earth’s history. Particulate matter and a variety of different gases from volcanoes, forest fires, and decaying organic materials in oceans and swamps enter the atmosphere at irregular intervals, sometimes in amounts that have dramatic effects. Naturally produced “greenhouse” gases, such as methane from plant decay, may have contributed significantly to periods of global warming in the past. Carbon dioxide and water vapor react to form carbonic acid, which makes rain slightly acidic even without pollution from other sources. Naturally produced pollutants are present in greater amounts than those of human origin. However, they do not present as serious a problem as man-made pollutants because they are not concentrated over large cities and many are less harmful than man-made pollutants. Man-made pollutants result primarily from burning fossil fuels (coal, gas, and oil) and other materials. Fossil fuels are burned in power plants that generate electricity, in many industrial plants, in home furnaces, and in cars, trucks, buses, and airplanes. Scientists estimate that about three-quarters of all air pollutants come from burning fossil fuels. The remaining pollutants largely result from industrial processes other than fuel burning, incinerating solid wastes, forest and agricultural fires, and the evaporation of chemicals. The following table summarizes the major sources of six primary air pollutants which are emitted in large quantities. Major Sources of Primary Air Pollutants Type of Pollutant Symbol Major Sources Carbon Dioxide CO2 Combustion of fossil fuels Carbon Monoxide CO Incomplete combustion of fossil fuels, mostly from vehicles Hydrocarbons HC Combustion of fossil fuels; petroleum refineries; petrochemical plants Nitrogen Oxides NOx Transportation vehicles; power plants Particulates Various Sulfur Oxides SOx Automobile exhaust; oil refining; coal-fired power plants; farming and construction operations Combustion of sulfur-containing fuels, especially coal Factors Affecting Air Pollution Many factors affect the type and the degree of air pollution found at a given place. Those over which people have relatively little control are climate, weather, wind patterns, and topography. These determine whether pollutants will accumulate or disperse. For example, a city on a plain is less likely to experience a buildup of pollution than is a city in a valley. Unusual weather can alter the normal patterns of pollutant dispersal. A temperature inversion magnifies the effects of air pollution. Typically, air temperature decreases with distance from the earth’s surface. However, if a stationary layer of warm, dry air exists over a region, cool air below – and any pollutants it contains – will be blocked in. The air pollutants generated in one place may have their most serious effect in areas hundreds of kilometers away. Since chemical reactions that produce air pollution can take hours or even days, it is not unusual that air currents have carried the pollutants away from the source city by this time. For example, Connecticut and parts of Massachusetts suffer from heavy smog due to pollutants originating from New York City. Similarly, the common occurrence of acid rain in New England and eastern Canada is caused by sulfurous emissions from power plants further west. Other factors that affect the type and the degree of air pollution at a given place are the levels of urbanization and industrialization. In general, the large and more industrialized a city is, the more pollution it generates. A city discharges more pollutants as population density, traffic density, type and density of industries, and living necessities increase. Types of Air Pollution Acid Rain Occurs when pollutants (primarily sulfur oxides and nitrogen oxides) created from the burning of fossil fuels chemically change as they are being transferred through the atmosphere and then fall back to the earth as acidic rain, snow, fog, or dust. The chemical change can be simplified into the following reactions: SOx + water vapour →sulfuric acid NOx + water vapour →nitric acid Two-thirds of acid rain is produced by sulfur dioxide. Nitrogen oxides are responsible for the remaining onethird. As described previously, one the pollutants are airborne, winds can carry them hundreds of kilometers away from the area in which they were produced. Seeing as most of the prevailing winds in North America are westerlies, much of the acid rain that falls on the eastern Canada originates in the American Midwest. The effects of acid rain can be seen on and off land. Soils are acidified, causing many nutrients to be leached out. Furthermore, microorganisms in soil, vital for breaking down organic matter and recycling nutrients through the ecosystem, can be killed. Such effects have devastated forests in areas of eastern Canada and the United States. As for the aquatic effects, it doesn’t take much of an increase in acidity of a lake or stream before the early reproductive stages of fish are affected. Not only does this cause a drastic decline in fish numbers in hundreds of lakes and streams in Canada and the U.S., but the food chain is also disrupted as acidification kills the plants and insects upon which fish feed. Photochemical Smog and Ozone Photochemical smog is produced when nitrogen oxides react with the oxygen present in water vapor in the air to form nitrogen dioxide. In the presence of sunlight, nitrogen dioxide reacts with hydrocarbons from automobile exhausts and industry to form new compounds, such as ozone (O3). Warm, dry weather and poor air circulation promote ozone formation (refer back to “temperature inversions” above). This is why smog alerts are summertime occurrences in cities. Areas most affected by smog are both demographical and physical in nature. Because the primary sources of nitrogen oxides are vehicles and industry, smog tends to be an urban problem. If the city is located in a warm, sunny climate region, the likelihood of ground-level ozone developing is greatly increased. Topography also plays a key role. Valley cities that are encircled by mountains are prone to temperature inversions. When temperature inversions occur, the pollutants are effectively trapped, unable to escape to the stratosphere. Although smog originates in urban industrial centers, it can affect areas downwind from them. Human health effects (asthma, pneumonia, and emphysema) due to ozone exposure and polluted air have been documented in urban and non-urban settings. Questions: 1. Fill in the following chart comparing natural and anthropogenic sources of air pollutants. [5 marks] NATURAL ANTHROPOGENIC Sources: Degree of Severity: Areas in which they are concentrated: 2. The “Major Sources of Primary Air Pollutants” table sites fossil fuels as a major source of many of the different pollutants. Go online to find two alternatives to fossil fuels and explain why your examples are better for the environment. [4 marks] 3. Research one of the following cities and briefly explain why it has an air pollution problem and some of the effects (human, vegetation, water) associated with the pollution. [6 marks] Choose from: Donora, Pennsylvania Las Angeles, California Denver, Colorado 4. Check out the following website: http://airqualityontario.com/press/smog_advisories.php. a. Look at the number of smog advisories and smog days for the province. Is there a trend? Why was there 53 smog days in 2005 (hint: do some additional research as to what the weather was like during this time). [4 marks] b. Browse through the years (2003 to 2014). Smog advisories and smog days are further defined by region. Which regions have typically had a greater number of smog advisories/days? Which had fewer? Can you see a trend? [4 marks] 5. The impacts acid rain has on forests and water were explained earlier. What effect can acid rain have on cities? [4 marks] 6. The Advisory Committee on the Environment to the City of London was asked to consider the “Emergency Recommendation for Smog Days Bylaw” in the Spring of 2008. The recommendations included considerations regarding the following: Open air burning (e.g. any outside fire) – when a smog alert has been declared, open air burning is strictly prohibited Anti-idling – zero idling on smog days Drive-Thru closures – all drive-thrus to close on any given smog day Free transit – City of London to provide free transit or “Pay What You Can” on all smog days Law equipment – zero operation of fuel powered lawn equipment on smog days In a paragraph, answer the following points [8 marks]: - Do you agree with the above recommendations? - Do you believe Hamilton should also try to meet these recommendations - If you agree, add two more recommendations to the list and explain why they would be beneficial to the Cities of London or Hamilton - If you do not agree, explain why, specifically addressing two of the recommendations and why they are unreasonable










